- Department of Neurosurgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
- Department of Diagnostic and Interventional Radiology, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
Correspondence Address:
Takao Tsurubuchi, Department of Neurosurgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
DOI:10.25259/SNI_636_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hiroki Karita1, Takao Tsurubuchi1, Taishi Amano2, Takao Koiso1, Noriaki Sakamoto1, Eiichi Ishikawa1. Spinal cord diffuse midline glioma with postoperative acute swelling: A case report and review of literature. 13-Oct-2023;14:360
How to cite this URL: Hiroki Karita1, Takao Tsurubuchi1, Taishi Amano2, Takao Koiso1, Noriaki Sakamoto1, Eiichi Ishikawa1. Spinal cord diffuse midline glioma with postoperative acute swelling: A case report and review of literature. 13-Oct-2023;14:360. Available from: https://surgicalneurologyint.com/surgicalint-articles/12597/
Abstract
Background: H3K27-altered diffuse midline glioma (DMG) is a newly classified disease according to the 5th edition of the World Health Organization classification of the central nervous system tumors. However, little is known about its progression pattern and the timing of surgical intervention, especially regarding spinal cord lesions.
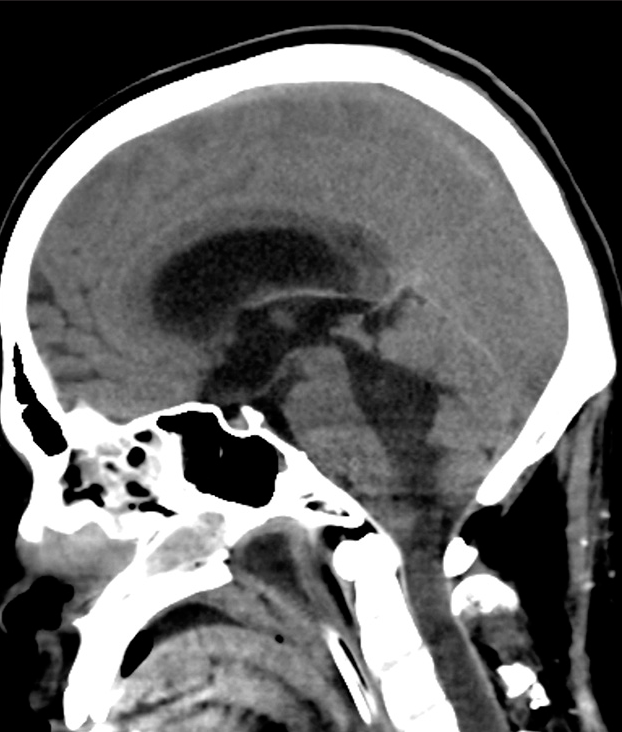
Case Description: A 26-year-old man presented with rapid muscle weakness progression in both upper and lower extremities and urinary dysfunction. Magnetic resonance imaging showed diffuse swelling of the cervicothoracic spinal cord. He underwent decompressive laminectomy with expansive duroplasty and tumor biopsy. The surgical specimen revealed DMG. Immediately after surgery, deterioration of limb paresis was observed, and the patient developed respiratory failure the day after surgery. Head-and-neck computed tomography on the 7th day after surgery showed spinal cord swelling and acute obstructive hydrocephalus.
Conclusion: We report a rare case of a spinal DMG with acute postoperative swelling. Neurological deterioration in patients with spinal cord DMG is often exacerbated, so it is essential to suspect DMG at an early stage based on neuroimaging, and if surgery is performed on the edematous spinal cord, further rapid swelling can occur, as in the present case.
Keywords: Cervical tumor, Diffuse midline glioma, H3K27-altered, H3K27M
INTRODUCTION
The histone H3-K27M mutation was first reported as causative for malignant gliomas, including brainstem gliomas, in 2012.[
Very few spinal cord DMG cases with rapid neurological deterioration have been reported.[
CASE REPORT
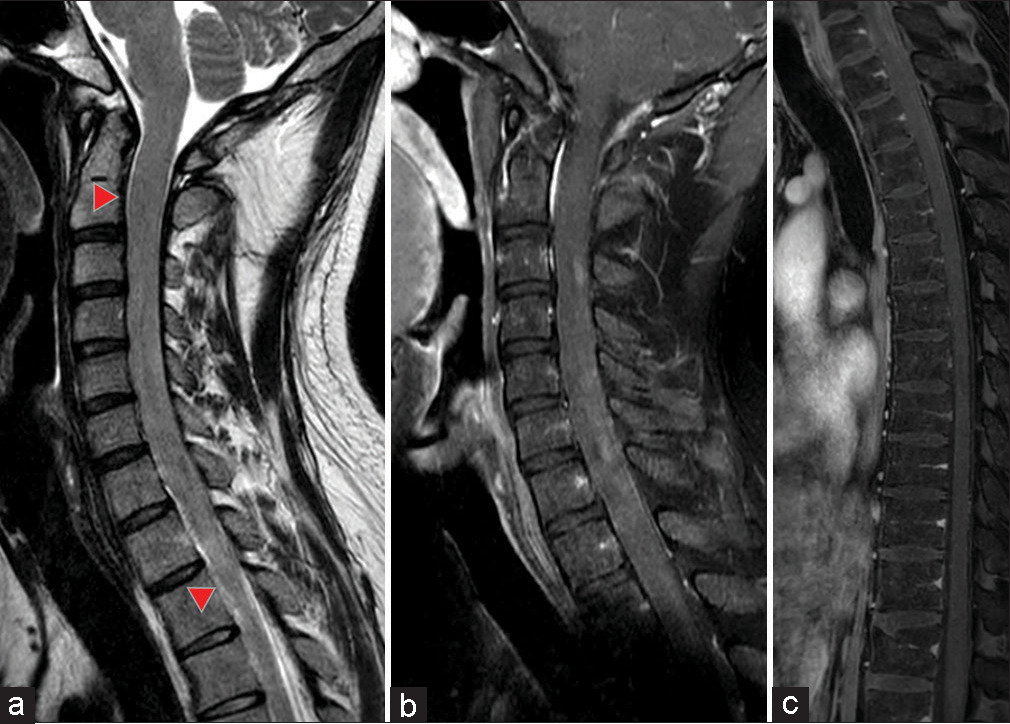
A 26-year-old man was diagnosed with an autism spectrum disorder in childhood and had been walking on tiptoes. At the age of 18, he started holding on to something when he stood, and 12 months ago, he began to drag his feet, gradually becoming unable to stand up straight, and developed frequent urination. Although MR imaging revealed a cervical cord enlargement, it was judged to have no pathological significance, and the patient was followed up by MR imaging at that time. Three months ago, he was referred to our hospital, complaining of urinary retention and progressive right upper limb movement disorder. Steroid pulse therapy was applied as a demyelinating disease was suspected. However, 1 month ago, the upper limb paralysis worsened. Due to the rapid progression of the symptoms, he was referred to our department. At the examination, the patient’s consciousness level was JCS I-3, and both upper and lower extremities showed severe paresis. Cervical T2-weighted MR imaging showed diffuse spinal cord swelling from the C2 to the Th2 level, and gadolinium-enhanced T1 images showed heterogeneous enhancement of the swollen spinal cord [
Figure 1:
Preoperative gadolinium contrast-enhanced magnetic resonance imaging images of the spinal cord. (a) T2-weighted sagittal image. Spinal cord enlargement is seen from C2 to Th2 levels (red arrowhead), with no spinal fluid cavity signal identified. (b and c) Sagittal T1-weighted images of the whole spinal cord with gadolinium contrast enhancement. Diffuse patchy enhancement is evident.
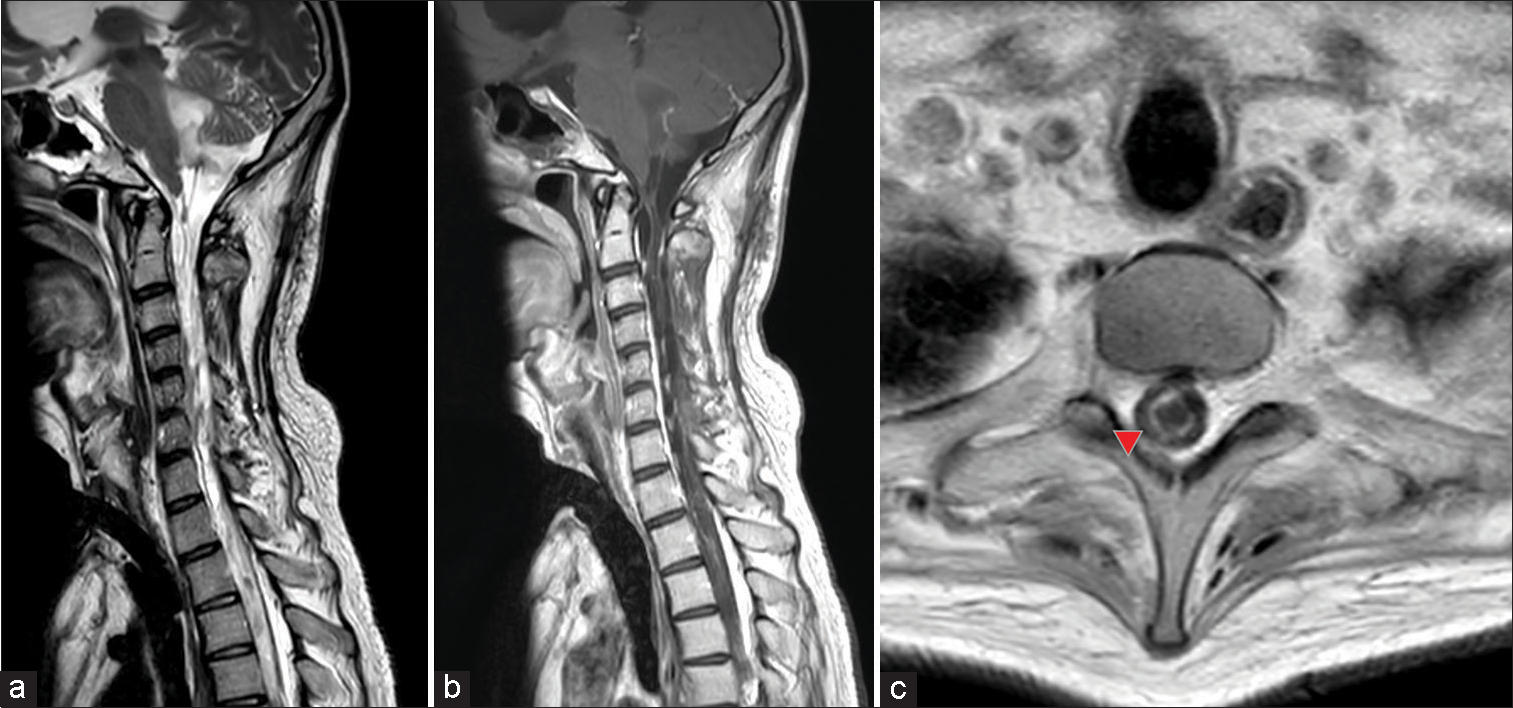
Figure 3:
Cervical gadolinium contrast-enhanced magnetic resonance (MR) imaging. (a) T2-weighted sagittal image showing severe atrophy of the spinal cord with little or no normal spinal cord tissue present. A liquid mass was observed in the fourth ventricle, suggesting a tumor with liquefaction changes. (b) Gadolinium-contrasted T1-weighted sagittal image. Partial contrast enhancement diffusely presented in the spinal cord, with spinal cord dissemination or residual tumor suspected. (c) Syringomyelia was observed on the axial T1-weighted plain MR image (red arrowhead).
DISCUSSION
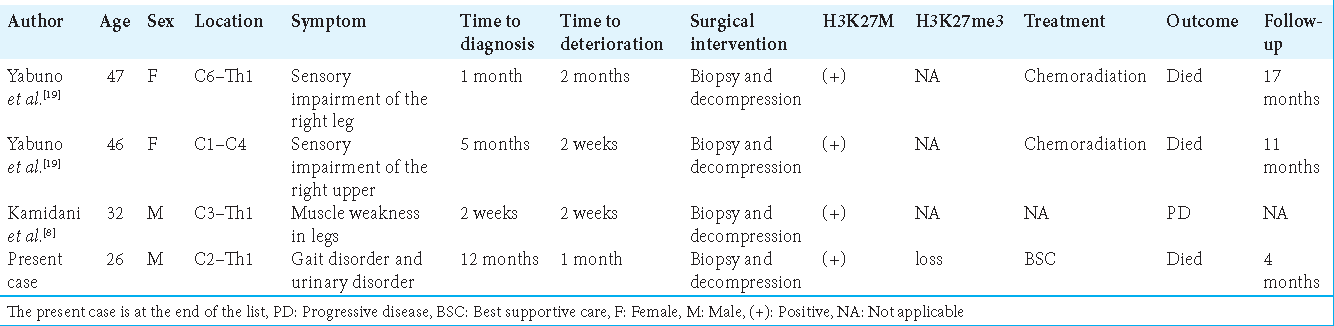
There have been few reports on H3K27-altered spinal cord DMG where acute deterioration in such a short period of time occurred. We summarized the reported cases, as shown in
The preoperative radiological diagnosis of spinal cord DMGs is difficult.[
In our case, there was considerable postoperative swelling of the cervical spinal cord. The causative mechanism of acute disease deterioration might be the rapid progression of the spinal cord swelling around the craniovertebral junction even after the decompression. Preoperative MR imaging showed the progression of the diffuse spinal cord swelling from the medulla oblongata level to the upper thoracic spine. It was assumed that decompression from C2 to C7 was insufficient to relieve the tension of the swollen spinal cord, resulting in obstructive hydrocephalus due to its upward herniation. Acute hydrocephalus caused by upward herniation has never been reported in spinal cord DMG, but it has been previously reported in hypertensive encephalopathy patients with the same mechanism as in our case.[
Furthermore, 2 months later, a follow-up contrast-enhanced MR imaging revealed signs of multiple syrinxes. Spinal syrinx (syringomyelia) usually develops after spinal trauma, in Chiari malformation, other head-and-neck abnormalities, and meningitis.[
CONCLUSION
We reported a rare case of acute spinal cord swelling in DMG after decompressive laminectomy with expansive duroplasty and tumor biopsy. The neurological deterioration in spinal cord DMG patients is often exacerbated, so it is essential to suspect DMG at an early stage based on neuroimaging, and if surgery is performed on the edematous spinal cord, further rapid swelling can occur, as in our case. It is important to monitor the symptoms closely and make a critical decision about the tumor biopsy with decompressive laminectomy before the deterioration of the patient’s condition.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial Support and Sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The authors would like to thank Alexander Zaboronok of the Department of Neurosurgery, Institute of Medicine, the University of Tsukuba, for professional and language revision.
References
1. Adamson DC, Dimitrov DF, Bronec PR. Upward transtentorial herniation, hydrocephalus, and cerebellar edema in hypertensive encephalopathy. Neurologist. 2005. 11: 171-5
2. Aihara K, Mukasa A, Gotoh K, Saito K, Nagae G, Tsuji S. H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro Oncol. 2014. 16: 140-6
3. Badri M, Gader G, Bahri K, Zammel I. Cervicothoracic syringomyelia caused by cervical spinal stenosis: Case report and literature review. Surg Neurol Int. 2017. 8: 288
4. Bhagavathula Venkata SS, Arimappamagan A, Lafazanos S, Pruthi N. Syringomyelia secondary to cervical spondylosis: Case report and review of literature. J Neurosci Rural Pract. 2014. 5: S78-82
5. Dormegny L, Chibbaro S, Ganau M, Santin M, Kremer L, Proust F. Biopsying a spinal cord lesion: A diagnostic dilemma. Case report and review of literature. Neurochirurgie. 2018. 64: 425-30
6. Greitz D. Unraveling the riddle of syringomyelia. Neurosurg Rev. 2006. 29: 251-63 discussion 264
7. Jung JS, Choi YS, Ahn SS, Yi S, Kim SH, Lee SK. Differentiation between spinal cord diffuse midline glioma with histone H3 K27M mutation and wild type: Comparative magnetic resonance imaging. Neuroradiology. 2019. 61: 313-22
8. Kamidani R, Okada H, Yasuda R, Yoshida T, Kusuzawa K, Ichihashi M. Diffuse midline glioma in the spinal cord with rapid respiratory deterioration. Acute Med Surg. 2020. 7: e500
9. Kimura R, Park YS, Nakase H, Sakaki T. Syringomyelia caused by cervical spondylosis. Acta Neurochir (Wien). 2004. 146: 175-8
10. Kurokawa R, Kurokawa M, Baba A, Ota Y, Pinarbasi E, Camelo-Piragua S. Major changes in 2021 world health organization classification of central nervous system tumors. Radiographics. 2022. 42: 1474-93
11. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
12. Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012. 482: 226-31
13. Shih RY, Koeller KK. Intramedullary masses of the spinal cord: Radiologic-pathologic correlation. Radiographics. 2020. 40: 1125-45
14. Solomon DA, Wood MD, Tihan T, Bollen AW, Gupta N, Phillips JJ. Diffuse midline gliomas with histone H3-K27M mutation: A series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 2016. 26: 569-80
15. Thrower BW. Clinically isolated syndromes: Predicting and delaying multiple sclerosis. Neurology. 2007. 68: S12-5
16. Won YL, Choi Y, Yuh WT, Kwon SW, Kim CH, Yang SH. Validity of magnetic resonance imaging (MRI) in the primary spinal cord tumors in routine clinical setting. Sci Rep. 2022. 12: 10151
17. Wingerchuk DM, Lucchinetti CF. Comparative immunopathogenesis of acute disseminated encephalomyelitis, neuromyelitis optica, and multiple sclerosis. Curr Opin Neurol. 2007. 20: 343-50
18. Wu G, Broniscer A, McEachron TA, Lu C, Paugh BS, Becksfort J. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012. 44: 251-3
19. Yabuno S, Kawauchi S, Umakoshi M, Uneda A, Fujii K, Ishida J. Spinal cord diffuse midline glioma, H3K27M-mutant effectively treated with bevacizumab: A report of two cases. NMC Case Rep J. 2021. 8: 505-11