- Department of Neurosurgery Pedro Ernesto University Hospital, Rio de Janeiro State University, Rio de Janeiro, Brazil.
- Department of Endocrine, Pedro Ernesto University Hospital, Rio de Janeiro State University, Rio de Janeiro, Brazil.
Correspondence Address:
Jefferson Trivino-Sanchez, Department of Neurosurgery, Pedro Ernesto University Hospital, Rio de Janeiro State University, Rio de Janeiro, Brazil.
DOI:10.25259/SNI_654_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jefferson Trivino-Sanchez1, Pedro Henrique Costa Ferreira-Pinto1, Elington Lannes Simões1, Felipe Gonçalves Carvalho1, Diego Rodrigues Menezes1, Thaina Zanon Cruz1, Julia Pereira Muniz Pontes1, Ana Beatriz Winter Tavares2, Flavio Nigri1. Spinal dural arteriovenous fistula rupture after Rathke’s cleft cyst endoscopic resection: Case report and literature review. 06-Sep-2021;12:455
How to cite this URL: Jefferson Trivino-Sanchez1, Pedro Henrique Costa Ferreira-Pinto1, Elington Lannes Simões1, Felipe Gonçalves Carvalho1, Diego Rodrigues Menezes1, Thaina Zanon Cruz1, Julia Pereira Muniz Pontes1, Ana Beatriz Winter Tavares2, Flavio Nigri1. Spinal dural arteriovenous fistula rupture after Rathke’s cleft cyst endoscopic resection: Case report and literature review. 06-Sep-2021;12:455. Available from: https://surgicalneurologyint.com/surgicalint-articles/11086/
Abstract
Background: Spinal dural arteriovenous fistula (SDAVF) is the most frequent vascular malformation of the spine and accounts for approximately 70% of all vascular spinal malformations. In rare cases, SDAVF rupture and subsequent subarachnoid hemorrhage or intramedullary hematoma may occur. The aim of this article is to present a fatal case of SDAVF rupture after a Rathke’s cleft cyst (RCC) endoscopic resection.
Case Description: An 80-year-old female was referred to our hospital with a clinical presentation of bilateral reduction in visual acuity, bitemporal hemianopsia, and sellar magnetic resonance imaging (MRI) highly suggestive of RCC. After the first endonasal endoscopic surgery, the cyst was partially removed and vision improved. No signs of cerebrospinal fluid (CSF) leak were observed. After 1 year, the patient returned because of RCC recurrence and decreased visual acuity. In the second procedure, the lesion was totally resected and CSF leak was observed. A nasoseptal flap was rotated to cover the skull base defect. The patient developed subtle paraparesis followed by paraplegia on the 4th postoperative day. The dorsal spine MRI revealed a T3-T4 intramedullary hematoma. A dorsal laminectomy was performed and a SDAVF was observed. During microsurgery, at the right T3 nerve root level, an arteriovenous shunting point was identified, coagulated, and divided. The intramedullary hematoma was evacuated. The patient developed neurogenic and septic shock and died.
Conclusion: Venous hypertension, venous wall fragility, and venous thrombosis seem to be the main factors involved in SDAVF rupture. In this particular case, reduction of the extravascular pressure and sudden variation in the pressure gradient caused by sustained CSF leak, also appeared to play an important role in SDAVF rupture. It may represent one more complication related to radical resection of RCC.
Keywords: Cerebrospinal fluid fistula, Rathke’s cleft cyst, Rupture, Spinal dural arteriovenous fistula, Transsphenoidal resection
INTRODUCTION
A spinal dural arteriovenous fistula (SDAVF) is an abnormal connection between arteries and veins located near the dura mater.[
CLINICAL PRESENTATION
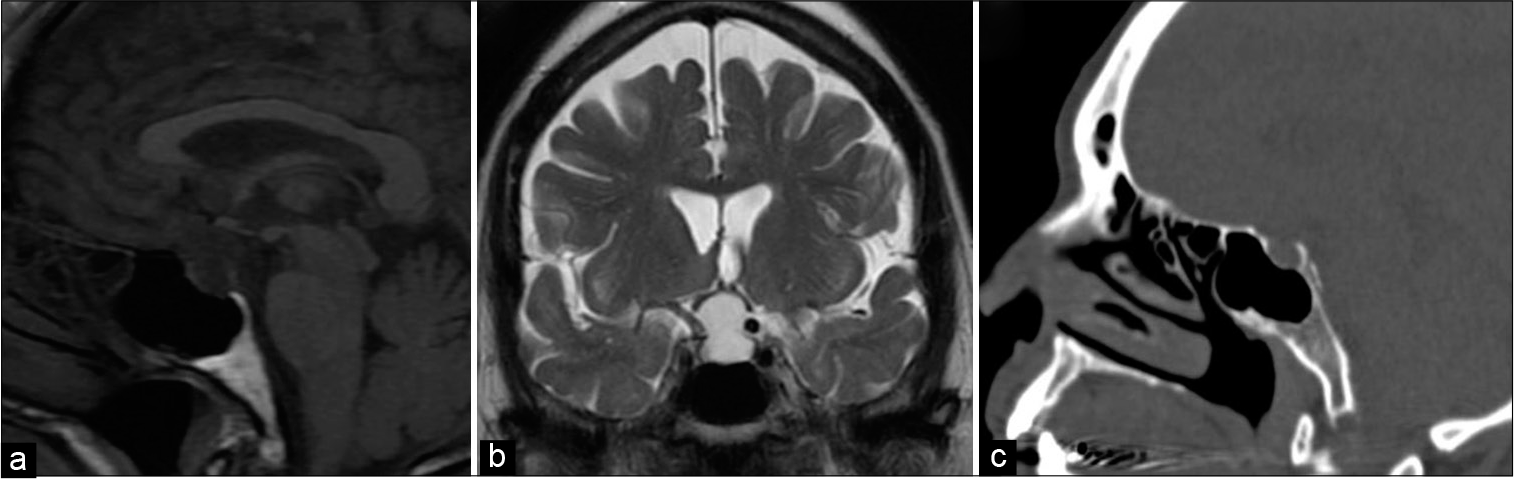
An 80-year-old female was referred to our tertiary hospital for surgical treatment of a recurrent RCC. The first endoscopic surgery, performed 2 years previously in the same institution, consisted of cyst fenestration, and partial cyst wall resection. The pedicled nasoseptal flap technique[
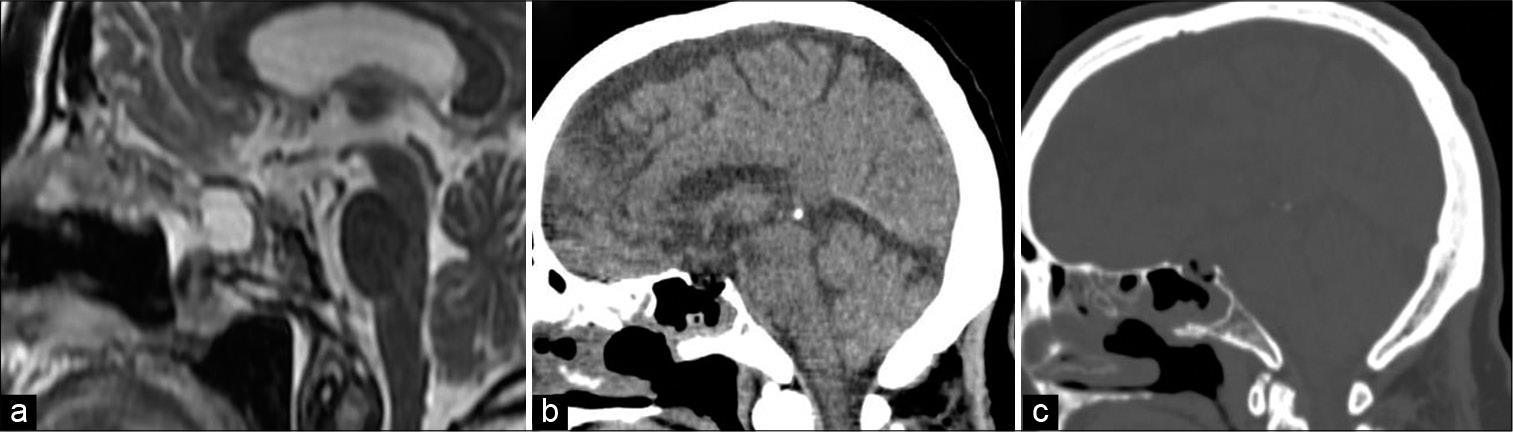
One year later, the patient presented recurrence of visual disturbance and bifrontal headaches. The neurologic examination revealed anisocoric pupils, with fixed mydriasis in the left eye and bitemporal hemianopsia. A new endoscopic transnasal transsphenoidal surgery was performed and a gross total resection (GTR) was achieved. However, during cyst dissection, a CSF leak was observed. Resurface of the skull base with a nasoseptal flap was performed. No hypertensive episodes were observed during the procedure. Postoperative sellar MRI confirmed total resection [
Figure 2:
Postoperative sellar MRI and CT scan. (a) MRI - T2-weighted sagittal slice demonstrating empty sellar space after Rathke’s cleft cyst gross total resection and nasoseptal flap closure. (b) CT sagittal slice shows minimum pneumoencephalon in sellar region after endoscopic procedure (c) Bone window CT sagittal slice - demonstrating absence of bone coverage on sellar floor.
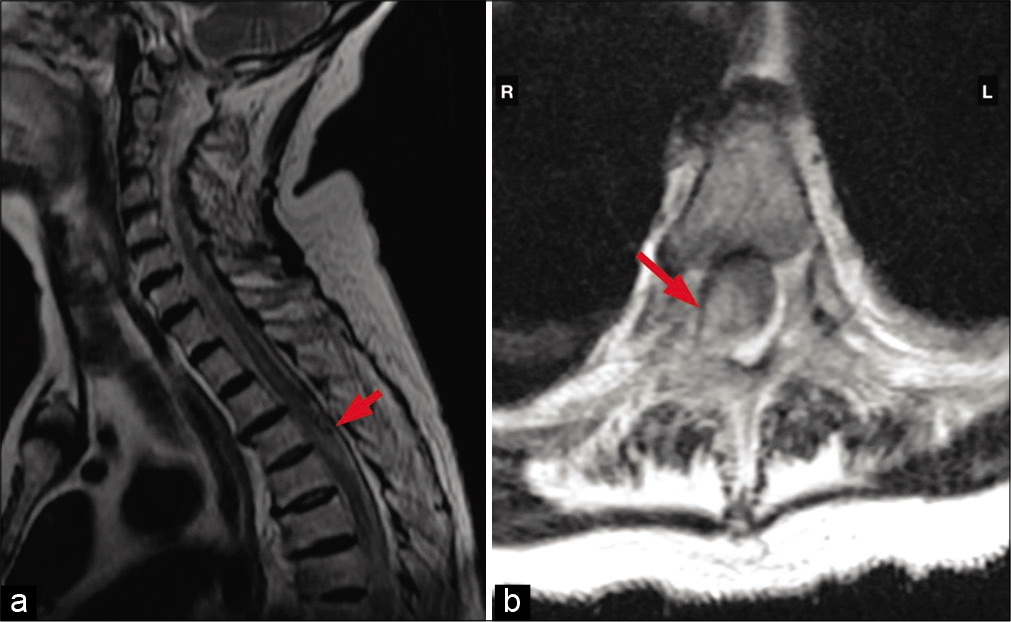
The patient developed subtle paraparesis followed by paraplegia on the 4th postoperative day. Dorsal spine MRI revealed a T3-T4 intramedullary lesion. The lesion was hyperintense on T2 and most notably in the right side of the spinal cord on the axial plane [
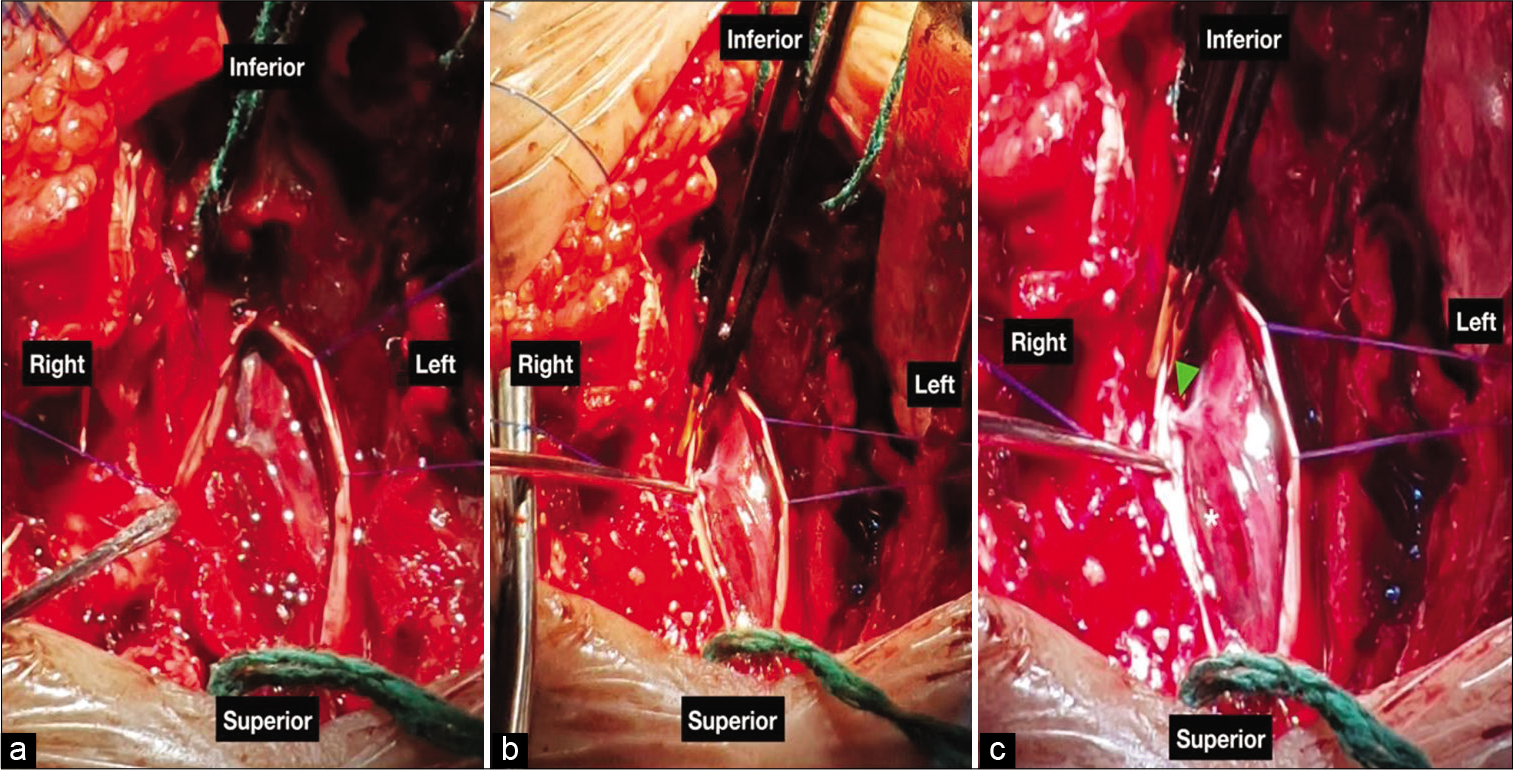
Figure 4:
Spinal dural arteriovenous fistula (SDAVF) microsurgery. (a) After dural opening, an important medullary hematoma associated with SDAVF was identified. (b) After blood aspiration, the SDAVF became more evident. (c) The spinal hematoma was aspirated (asterix). After identification of the right T3 artery feeder and venous portion, the shunt was coagulated and cut.
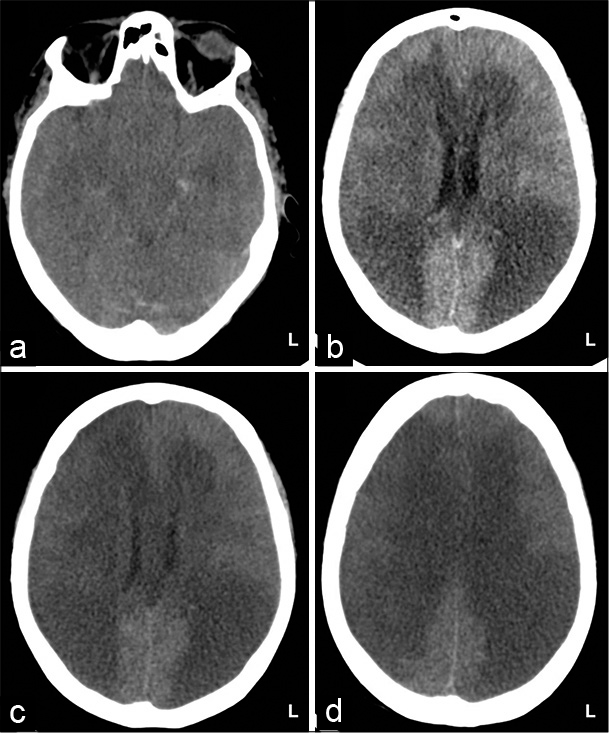
Immediately after surgery, the patient presented neurogenic shock needing high doses of norepinephrine. She was admitted to the intensive care unit. From this moment, no signs of CSF rhinorrhea were observed. Four days after laminectomy, there was a progressive worsening of neurogenic shock associated with septic shock due to pneumonia requiring higher doses of vasoactive amines. Mean arterial pressure ranged between 50 and 60 mmHg despite high doses of norepinephrine. Ten days after laminectomy, the patient developed a hypoxic encephalopathy resulting in brain death. The brain CT demonstrated a diffuse white matter hypodensity [
DISCUSSION
RCC
RCC are sellar or suprasellar non-neoplastic cystic lesions arising from remnants of Rathke’s pouch.[
Unfortunately, because decompression alone leaves the cyst wall intact, it has been associated with a higher level of cyst scarring and re-accumulation of the cyst content. Alternative measures have been developed to stabilize the cystic cavity, allowing continual drainage, and avoiding cystic regrowth. One technique that has been popularized is the cyst marsupialization, where the cyst wall is widely opened and the cavity is opened to drain direct into the sphenoid sinus.[
SDAVF
Dorsal SDAVF (Type I according Di Chiro and Wener[
Patients with SDAVF often present non-specific clinical features that are related to progressive myelopathy. The most common clinical presentations are progressive pain, lower extremity weakness or sensory changes, and sphincter dysfunction.[
Because the lower thoracic region has relatively fewer venous outflow channels compared with the cervical region,[
Diagnosis first relies on MRI that shows central cord hyperintensity on T2-weighted images and increased perimedullary flow voids, most commonly dorsal to the cord.[
SDAVFs rarely bleed.[
In our particular case, these mentioned factors seem to have contributed to SDAVF rupture based on surgical findings (e.g., the single arterialized and thrombosed vein). However, no arterial hypertension episode was noted during the patient’s clinical course and corticosteroid therapy was not administrated, which could justify a state of hypercortisolism and SDAVF rupture. In addition, we must consider the fact that there was a sustained CSF fistula with a probable decrease in intracranial and subarachnoid space pressures. The CSF hypotension and subsequent decreased extravascular pressure generating a sudden variation of intra and extravascular pressures (pressure gradient) may also play a key role in the SDAVF bleeding. It is the 1st time that this association is mentioned. Unfortunately, we cannot categorically state whether the SDAVF rupture was due to extravascular factors caused by the CSF fistula or if it happened coincidentally.
Other factors involving SDAVF rupture were also studied by other groups. In a recent review, Yue et al.[
With the evolution of endovascular therapy, catheter-based therapy is often used as the treatment or as a component of treatment of vascular malformations of the spine. Many practitioners advocate endovascular treatment of type I SDAVFs and report good clinical outcomes in some cases.[
CONCLUSION
Venous hypertension, venous wall fragility, and venous thrombosis seem to be the main factors involved in SDAVF rupture. In this particular case, reduction of the extravascular pressure and sudden variation in the pressure gradient caused by sustained CSF leak, also appeared to play an important role in SDAVF rupture. It may represent one more complication related to radical resection of RCC.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Center of High Complexity Neurosurgery Intern Patients (NIPNAC) from Rio de Janeiro State Department of Health (SES-RJ).
Conflicts of interest
There are no conflicts of interest.
References
1. Andres RH, Barth A, Guzman R, Remonda L, El-Koussy M, Seiler RW. Endovascular and surgical treatment of spinal dural arteriovenous fistulas. Neuroradiology. 2008. 50: 869-76
2. Benveniste RJ, King WA, Walsh J, Lee JS, Naidich TP, Post KD. Surgery for Rathke cleft cysts: Technical considerations and outcomes. J Neurosurg. 2004. 101: 577-84
3. di Chiro G, Wener L. Angiography of the spinal cord. A review of contemporary techniques and applications. J Neurosurg. 1973. 39: 1-29
4. Hamdan A, Padmanabhan R. Intramedullary hemorrhage from a thoracolumbar dural arteriovenous fistula. Spine J. 2015. 15: e9-16
5. Han SJ, Rolston JD, Jahangiri A, Aghi MK. Rathke’s cleft cysts: Review of natural history and surgical outcomes. J Neurooncol. 2014. 117: 197-203
6. Hassler W, Thron A. Flow velocity and pressure measurements in spinal dural arteriovenous fistulas. Neurosurg Rev. 1994. 17: 29-36
7. Higgins DM, Meyer FB. Symptomatic Rathke cleft cysts: Extent of resection and surgical complications. Neurosurg Focus. 2011. 31: E2
8. Jeng Y, Chen DY, Hsu HL, Huang YL, Chen CJ, Tseng YC. Spinal dural arteriovenous fistula: Imaging features and its mimics. Korean J Radiol. 2015. 16: 1119-31
9. Kassam AB, Thomas A, Carrau RL, Snyderman CH, Vescan A, Prevedello D. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery. 2008. 63: ONS44-52
10. Koch C, Gottschalk S, Giese A. Dural arteriovenous fistula of the lumbar spine presenting with subarachnoid hemorrhage. Case report and review of the literature. J Neurosurg. 2004. 100: 385-91
11. Krings T, Geibprasert S. Spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2009. 30: 639-48
12. Kuan EC, Trent MS, Luu NN, Kohanski MA, Tong CC, O’Malley BW. Preventing restenosis of marsupialized Rathke cleft cysts using a nasoseptal flap lining. Laryngoscope. 2019. 129: 2258-61
13. Larkin S, Karavitaki N, Ansorge O. Rathke’s cleft cyst. Handb Clin Neurol. 2014. 124: 255-69
14. Lu VM, Ravindran K, Perry A, Graffeo CS, Dawood HY, van Gompel JJ. Recurrence of Rathke’s cleft cysts based on gross total resection of cyst wall: A meta-analysis. Neurosurg Rev. 2020. 43: 957-66
15. Marcus J, Schwarz J, Singh IP, Sigounas D, Knopman J, Gobin YP. Spinal dural arteriovenous fistulas: A review. Curr Atheroscler Rep. 2013. 15: 335
16. Minami M, Hanakita J, Takahashi T, Kitahama Y, Onoue S, Kino T. Spinal dural arteriovenous fistula with hematomyelia caused by intraparenchymal varix of draining vein. Spine J. 2009. 9: e15-9
17. Niu X, Liu H, Li J. Intramedullary hemorrhage from a thoracic dural arteriovenous fistula. Neurology. 2020. 95: 881-2
18. Schmidt KA, Huang JF, Black DF, Kaufmann TJ, Lanzino G, Kumar N. Spinal dural arteriovenous fistula with intramedullary cord hemorrhage. Neurol Clin Pract. 2014. 4: 486-9
19. Spetzler RF, Detwiler PW, Riina HA, Porter RW. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002. 96: 145-56
20. Trifanescu R, Ansorge O, Wass JA, Grossman AB, Karavitaki N. Rathke’s cleft cysts. Clin Endocrinol (Oxf). 2012. 76: 151-60
21. Vivekanandam V, Li V, Wu T, Dowling R, Roxburgh RH, McGuiness BJ. Cerebrospinal fluid cannot be used to distinguish inflammatory myelitis from congestive myelopathy due to spinal dural arteriovenous fistula: Case series. BMJ Neurol Open. 2019. 1: e000019
22. Yue H, Ling W, Ou Y, Chen H, Po Z, Wang B. Intracranial subarachnoid hemorrhage resulting from non-cervical spinal arteriovenous lesions: Analysis of possible cause of bleeding and literature review. Clin Neurol Neurosurg. 2019. 184: 105371
23. Zada G. Rathke cleft cysts: A review of clinical and surgical management. Neurosurg Focus. 2011. 31: E1
24. Zhu D, Zhao P, Lv N, Li Q, Fang Y, Li Z. Rupture risk of cerebral arteriovenous malformations during pregnancy and puerperium: A single-center experience and pooled data analysis. World Neurosurg. 2018. 111: e308-15