- Ghaly Neurosurgical Associates, Aurora, Illinois, USA
- Department of Neurosurgery, University of Illinois, Chicago, Illinois, USA
- Department of Anesthesiology, University of Illinois, Chicago, Illinois, USA
- Department of Anesthesiology, Advocate Illinois Masonic Medical Center, Chicago, Illinois, USA
Correspondence Address:
Ramsis F. Ghaly
Department of Anesthesiology, University of Illinois, Chicago, Illinois, USA
Department of Anesthesiology, Advocate Illinois Masonic Medical Center, Chicago, Illinois, USA
DOI:10.4103/2152-7806.191074
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ghaly RF, Lissounov A, Tverdohleb T, Kohanchi D, Candido KD, Knezevic NN. Spinal neuromodulation as a novel surgical option for failed back surgery syndrome following rhBMP exuberant bony growth in instrumented lumbar fusion: A case report and literature review. Surg Neurol Int 22-Sep-2016;7:
How to cite this URL: Ghaly RF, Lissounov A, Tverdohleb T, Kohanchi D, Candido KD, Knezevic NN. Spinal neuromodulation as a novel surgical option for failed back surgery syndrome following rhBMP exuberant bony growth in instrumented lumbar fusion: A case report and literature review. Surg Neurol Int 22-Sep-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/spinal-neuromodulation-novel-surgical-option-failed-back-surgery-syndrome-following-rhbmp-exuberant-bony-growth-instrumented-lumbar-fusion-case-report-literature-review/
Abstract
Background:Bone morphogenic protein (BMP) for instrumented lumbar fusion was approved in 2002, and since then has led to an increasing incidence of BMP-related neuropathic pain. These patients are usually resistant to conventional medical therapy and frequently undergo multiple surgical revisions without any pain relief.
Case Description:A 58-year-old male was referred to the author's outpatient clinic after four lumbar surgeries did not provide satisfactory pain relief. During his 10 years of suffering from low back pain after an injury, the patient was resistant to conventional and interventional treatment options. He was experiencing severe back pain rated 10/10, as well as right lower extremity pain, numbness, tingling, and motor deficits. Outside spine specialists had performed revision surgeries for BMP-related exuberant bone formation at L5–S1, which included the removal of the ipsilateral hardware and debridement of intradiscal and intraforamina heterotrophic exuberant bony formation. The author implanted the patient with a permanent continuous spinal cord stimulator, after which he achieved complete pain relief (0/10) and restoration of motor, sensory, autonomic, and sphincter functions.
Conclusion:This is the first reported case of restorative function with neuromodulation therapy in a BMP-induced postoperative complication, which is considered as a primarily inflammatory process, rather than nerve root compression due to exuberant bony formation. We hypothesize that neuromodulation may enhance blood flow and interfere with inflammatory processes, in addition to functioning by the accepted gate control theory mechanism. The neuromodulation therapy should be strongly considered as a therapeutic approach, even with confirmed BMP-induced postoperative radiculitis, rather than proposing multiple surgical revisions.
Keywords: Bone morphogenetic protein, exuberant bony formation, failed-back surgery syndrome, instrumented lumbar fusion, neuromodulation
INTRODUCTION
Since the introduction of recombinant human bone morphogenetic protein (rhBMP), there is a growing number of patients suffering from neuropathic pain as a result of local BMP-related nerve root compression by exuberant bony formation and localized inflammatory processes. At present, there are no good therapeutic options for relief of these patients’ suffering. The introduction of rhBMP (InFUSE, Medtronic Sofamor Danek, Memphis, TN, USA) did not anticipate the fact that the new bone formation would continue to grow without regard to nearby neurological structures. The new bone formation can be found at the disk level within foraminal structures and can continue to grow with a vengeance. These locations are notoriously difficult to access surgically, and the surgical removal of exuberant bony formation is extremely challenging.
Bone morphogenetic proteins are multifunctional growth factors that were originally identified in the 1960s.[
In 2002, the Food and Drug Administration approved InFUSE Bone/LT-CAGE™ Lumbar Tapered Fusion Device (Medtronic Sofamor Danek, Memphis, TN, USA) as a recombinant human bone morphogenetic protein-2 [rh-BMP-2] solution with a carrier for the BMP solution (an absorbable collagen sponge made of bovine type I collagen) and a temporary metallic tapered spinal fusion cage.[
Treatment for heterotopic ossification and BMP-related radiculitis includes revision decompression surgeries, epidural steroid injections, and high doses of opioid medications often with marginal improvement or none at all.[
We report, for the first time, the benefits of spinal neuromodulation using a spinal cord stimulator therapy to successfully alleviate and relive neuropathic pain caused by rhBMP-induced exuberant bony formation.
CASE PRESENTATION
A 58-year-old right hand-dominant gentleman presented to the author's outpatient pain clinic with a persistent severe low back pain and right lower extremity pain, numbness, tingling, and partial right foot drop which did not improve with conservative treatment and four back surgeries. The patient rated his pain as 10 out of 10 on an 11-point verbal pain rating scale. He described his pain as burning, sharp, shooting, stabbing, deep pain, increased by lifting, climbing, straining, sitting, walking, sneezing, and present even at rest. The patient had a history of uncontrolled insulin-dependent diabetes mellitus with the last measured blood glucose value of 273 mg/dL and hemoglobin A1c (HA1C) of 9.3%. In addition, he had tinnitus, erectile dysfunction, hypercholesterolemia, history of chronic pain syndrome, and chronic narcotic dependency. The review of organ systems was not significant for other conditions.
Physical exam was notable for a positive straight right leg raise at 5°; there was significant neuropathy below the knees and below the ankles bilaterally. Allodynia was noted in the right L5 nerve root distribution and a right foot drop with 3/5 strength on dorsiflexion was identified. Back flexibility was limited due to pain in both flexion (35°), and extension (10°). Walk was feasible with mild limping presence, however, no walking-assist devices were used.
He previously sustained a work-related injury approximately 10 years ago that presented with low back pain and radiculopathy. Initially, he presented to his primary care physician with symptoms of right, more than left lower extremity numbness, tingling, and radicular pain which followed the pattern of the L5 and S1 nerve root distributions. At that time, the magnetic resonance imaging (MRI) of his lumbar spine showed a mild disk bulge at the L5–S1 level, and no other significant findings. The patient underwent conservative treatment modalities over the course of several years, including physical therapy (multiple 6–10 week courses), myriad local/epidural/facet injections, and lumbar medial branch radiofrequency nerve ablations (>100), and increasing doses of narcotic medications (Hydrocodone–Acetaminophen: Sometimes even up to 12 pills a day, Morphine sulfate and Hydromorphone); all with minimal sustained relief. His quality of life started to deteriorate, his marital, social, and work life were greatly suffering and he discontinued his employment as a technician.
Surgical spine intervention was advised by other spine specialists which led to four surgeries in different hospitals as followed chronologically below during a period of five years. The first surgery consisted of right L5 hemilaminectomy, right L5–S1 foraminotomy with decompression of the nerve roots and microdiscectomy. Despite the surgery, right leg pain persisted. Several months post-surgery, a computed tomography (CT) scan of the lumbar spine with contrast showed mild disc bulges at L5–S1, left neural foraminal narrowing at the L4–L5 and L5–S1 levels and right L5–S1 foraminal narrowing [Figure
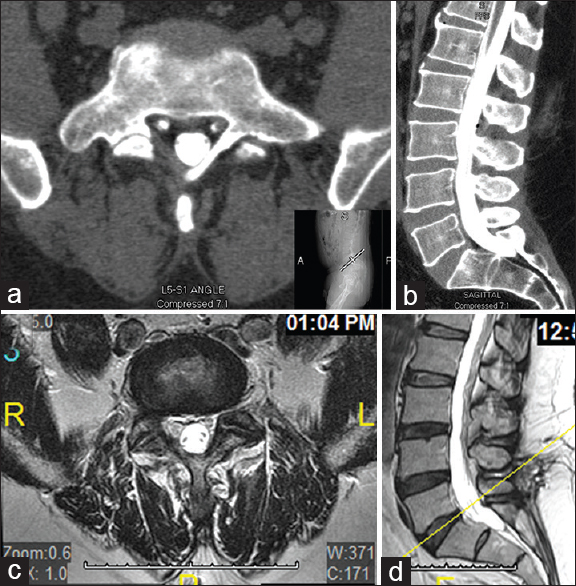
Figure 1
Imaging study after first surgery (right hemi-laminectomy and foraminotomy at L5–S1). Lumbar computed tomography (CT) myelogram during several month follow-up; (a) Transverse plane view at L5–S1; (b) Sagittal plane view. Lumbar Magnetic resonance imaging (MRI) during a 1-year follow-up; (c) Transverse plane view at L5–S1; (d) Sagittal plane view
The second surgery was performed after an extensive history and imaging work-up during a 1-year follow-up. Surgery completed a revision of the right L5–S1 discectomy and foraminotomy. The right L5–S1 nerve root was decompressed from the scar tissue of his previous surgery seen on MRI [Figure
The third surgery proceeded with an instrumented spinal fusion, which was based on left L5 laminar fracture, right L5 spondylolitic defect around the area of previous foraminotomy, and current L5 laminectomy. An instrumented fusion was accomplished using Legacy 5.5 rod system, allograft InFUSE (Medtronic Sofamor Danek, Memphis, TN, USA) with a transforaminal lumbar interbody fusion approach (TLIF). Despite the surgery, the patient had persistent pain at the right, more than left lower extremity, and lower back pain. His pain was 10 out of 10 and his quality of life continued to deteriorate thereafter. The patient was educated regarding behavioral modification programs and coping mechanisms.
On subsequent follow-up imaging studies after the third spine surgery, exuberant bony formation [Figure
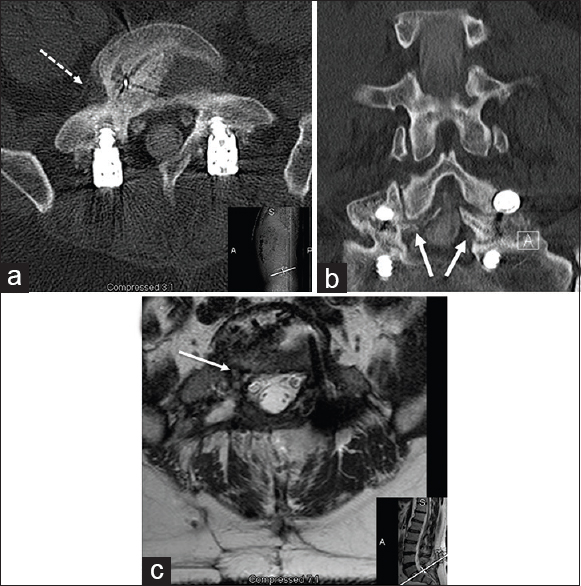
Figure 2
Imaging studies after third surgery (approximately 2 years after first surgery): An L5–S1 instrumented lumbar spine fusion with bilateral rod placement. Lumbar computed tomography (CT); (a) Transverse plane view at L5–S1 with BMP-induced exuberant intradiscal and intraforamina bony formation (white-dash arrow); (b) Coronal plane view with early encroachment of foramen bilaterally (white arrows); (c) Lumbar MRI at L5–S1 (transverse plane view) with evidence of heterotopic ossification at L5–S1. One-third and two-thirds of right L5–S1 neuroforamina occupied by exuberant bony growth. Progressive nerve root disfigurement (white arrow) and intracanalicular and intradiscal encroachment due to BMP-related exuberant bony formation and failure of surgical drilling
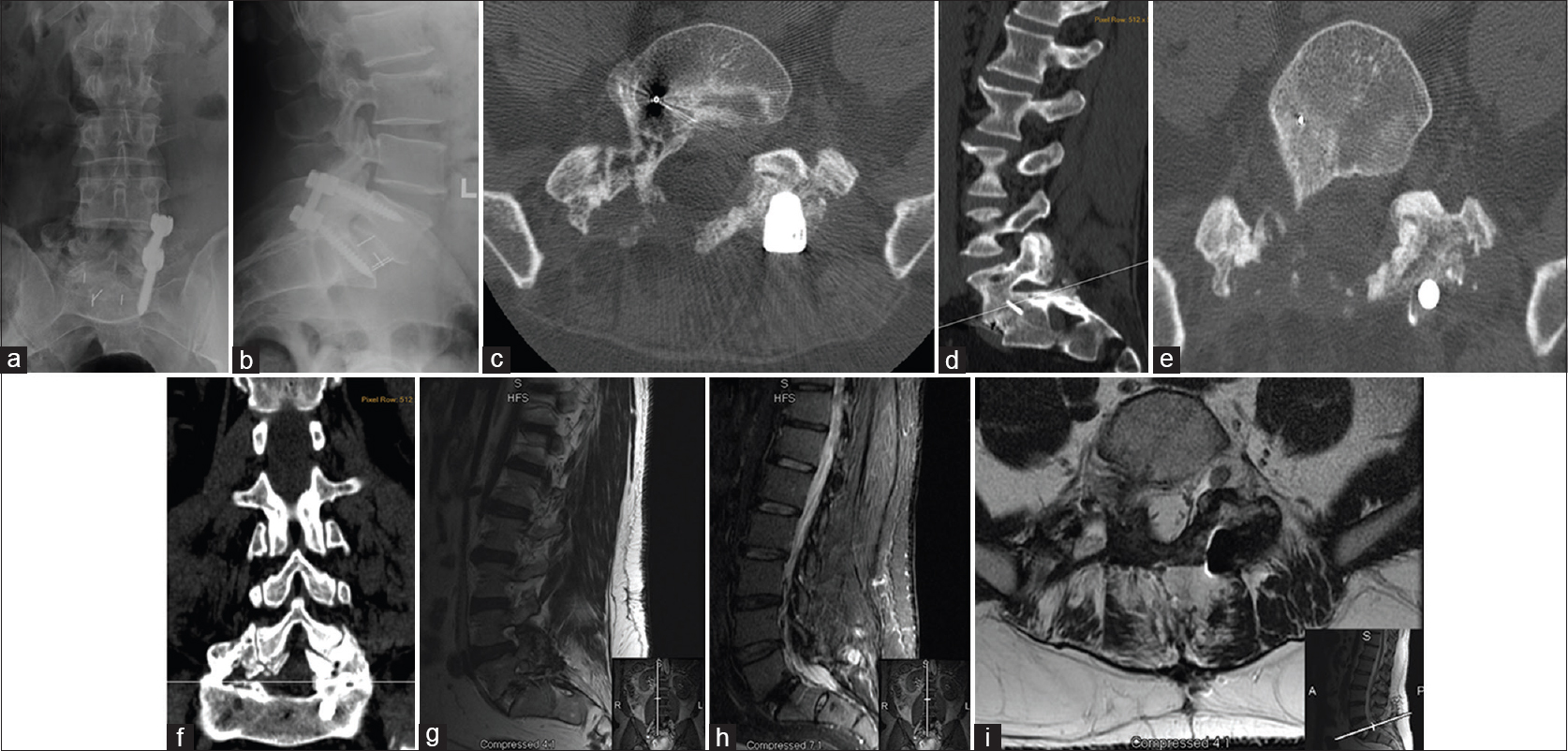
Figure 3
Lumbar imaging studies after 4th surgery (approximately 5 years after 1st surgery) - removal of hardware on the right and drilling out to remove osteophytic, exhotropic, heterotopic, and myxotrophe bone. Lumbar X-ray; (a) Antero-posterior (AP) view; (b) Lateral view—hardware is in place with open R-sided foramen; heterotopic bone growth is entangling all around the nerve root in the facet. Lumbar computed tomography (CT) confirmed heterotopic bone growth reaches and was able to grow in difficult places; (c) Transverse plane view; (d) Sagittal plane view showing remaining bone and entanglement of the right S1-nerve root, intradiscal and intraforaminal without respect for neural elements; (e) Transverse plane view; (f) Coronal plane view demonstrating post-surgical drilling of the exuberant bony formation when compared to right and left sides; bone growth is in continuity with disc post-lumbar fusion; significant amount of bone is drilled out on the right when compared to the left. (g) Sagittal plane view (T2 sequence); (h) Sagittal plane view (STIR sequence); (i) Transverse plane view (T2 sequence) showing foramen of new bone with incomplete bone removal after right screw removal and surgical drilling
Pre-neuromodulation procedure work-up
The patient was referred to the author for a second opinion. Before proceeding with the spinal cord stimulator (SCS) trial, the patient was referred to his primary care physician for education on diet, lifestyle, and management of his high serum blood glucose. In addition, the pre-surgical psychiatric evaluation confirmed appropriate psychological aptitude for successful implantation of the SCS. Furthermore, the patient was slowly tapered down to a low dose of narcotic medications, until he was completely removed from his opioid treatment regimen, which was repeatedly confirmed with repeated urine toxicology screenings. A comprehensive study was performed with detailed evaluation of his previous images. Imaging studies revealed no cord compression or disc herniation. CT and MRI scans [Figure
Neuromodulation intervention
The author's standard policy with neuromodulation therapy allows patients to explore options of all major neurostimulator manufactures (Boston Scientific, Medtronic, and St Jude Medical). The patient's preference was Medtronic's neurostimulator (Medtronic, INC, Minneapolis, MN, USA) that would permit future MRI utilization (Vectris SureScan MRI leads and SureScan MRI neurostimulator) and accommodate to changing body positions (RestoreSensor neurostimulator). A temporary trial using Medtronic percutaneous surgical leads was performed with proximal contacts at T9–T10. The trial was successful with an immediate pain reduction to 4 out of 10 from his baseline of 10 out of 10. Pleased with the trial outcome, the patient was implanted with the permanent double Medtronic Vectris SureScan MRI percutaneous leads at T8–T10 level, and connected to the IGP generator with a RestoreSensor SureScan MRI neurostimulator [Figure
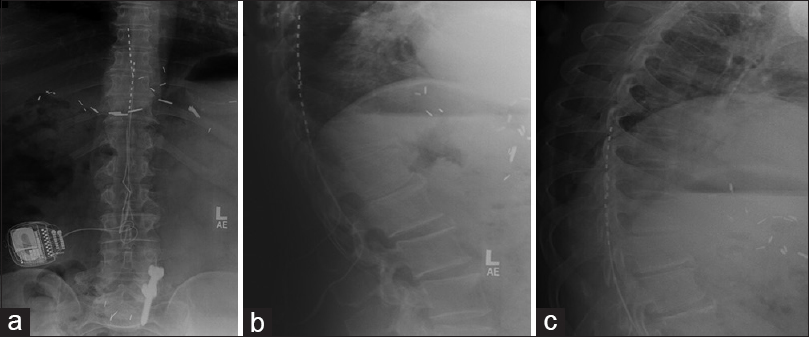
Figure 4
Lumbar X-ray after Spinal Cord Stimulator (SCS) implantation; (a) Anterior-posterior view showing lead placement in T8–T10 connected to the IPG generator with restore-sensor on the right; (b) Lateral view confirms leads position in the epidural space; (c) Lumbar X-ray at 2 months after SCS implantation with lateral views confirming position of leads in the epidural space and placement at T8–T10
Neuromodulation outcome
At the second year follow-up after neuromodulation therapy, the patient reported complete resolution of his low back pain and radicular symptoms with a consistent pain score of 0 out of 10. Furthermore, a complete restoration of motor, sensory, autonomic, and sphincteric function was observed using a continuous neurostimulator mode with a required 2-week generator recharge. The patient has been fully engaged in social activities as his mood and sleep have improved, and has reported an improvement of his marital relationship because erectile dysfunction has resolved. He has been weaned-off of all narcotics, neuropathic medications, and over the counter pain medications. Over the course of the neuromodulation therapy, he had not noted any fever, erythema, drainage, or swelling, and follow-up X-ray confirmed the placement of the leads without migration [
DISCUSSION
This case of multiple failed surgical spine fusions demonstrates a challenging scenario resulting from the pathophysiology of BMP-related complications (soft tissue swelling, postoperative radiculitis, and ectopic bone formation) that persisted in spite of numerous surgical revisions. A complete restoration of motor-sensory function with neuromodulation therapy, which is not considered to be a corrective procedure, should prompt further investigation to explain the complex mechanism of action of a SCS. We can hypothesize that the inflammatory process plays a vital etiologic role[
The exact mechanism of action of our treatment modality is difficult to determine. It has been hypothesized that continued nerve stimulation may increase blood flow, thereby minimizing potential ischemic insult and radicular symptoms associated with impingement.[
The efficacy and cost-effectiveness of a treatment method is an indisputable and important aspect in current clinical decision-making. Even so, while neuromodulation is not considered to be a corrective procedure, it is evident that an excellent symptom pain relief can be achieved in structurally-related lumbar injuries. North et al. studied the effectiveness and cost analysis for SCS versus reoperation in failed-back surgery syndrome (FBSS), and it was found that most of the patients randomized into reoperation crossed-over to SCS; 13 out of 21 patients (62%), whereas in the SCS group only 5 out of 19 (26%) crossed-over to operation.[
FBSS is a term commonly used to describe patients that, despite spine surgery, (laminectomy, discectomy, fusion) continue to have persistent back and/or leg pain.[
Our patient underwent extensive imaging studies and a multimodal treatment approach with complete pain resolution only after SCS implantation. This outcome may confirm the findings from studies on FBSS treatment, which compared the use of an SCS with conventional medical therapy (CMM) or repeat spine surgery.[
CONCLUSION
A complete recovery of neurological function and complete resolution of pain symptoms was achieved with neuromodulation intervention after 10 years of unsuccessful surgical revisions and conservative medical therapy. An inflammatory process is the primary pathoetiological factor in this case of BMP-induced neuropathic pain rather than the compressive nature of BMP-induced heterotrophic bony formation. This theory is most consistent as this case demonstrates successful symptom resolution with neuromodulation therapy and discontinuing pain medications (narcotics and OTC) entirely. This is a much more attractive approach when compared with multiple surgical revisions and dependency on pharmaceutical therapies. Therefore, we believe that the spinal neuromodulation should be a procedure of choice with confirmed BMP-related neuropathic pain.
Presentation at a meeting
Organization: North American Society of Neuromodulation (NANS) 19th Annual Meeting, Las Vegas, 10 December, 2015.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Atkinson L, Sundaraj SR, Brooker C, O’Callaghan J, Teddy P, Salmon J. Recommendations for patient selection in spinal cord stimulation. J Clin Neurosci. 2011. 18: 1295-1302
2. Baskin DS, Ryan P, Sonntag V, Westmark R, Widmayer MA. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine. 2003. 28: 1219-24
3. Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: A prospective, a randomized clinical plot trial: 2002 Volvo Award in clinical studies. Spine. 2002. 27: 2662-73
4. Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: A preliminary report. Spine. 2000. 25: 376-81
5. Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rh-BMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002. 15: 337-49
6. Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002. 27: 2396-408
7. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emergency safety concerns and lessons learned. Spine J. 2011. 11: 471-91
8. updated: August 6, 2015; cited January 18, 2016. Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm062000.htm.
9. Dawson E, Bae HW, Burkus JK, Stambough JL, Glassman SD. Recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge with an osteoconductive bulking agent in posterolateral arthrodesis with instrumentation. A prospective randomized trial. J Bone Joint Surg Am. 2009. 91: 1604-13
10. Dimar JR, Glassman SD, Burkus JK, Carreon LY. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine. 2006. 31: 2534-9
11. Dimar JR, Glassman SD, Burkus JK, Pryor PW, Hardacker JW, Carreon LY. Clinical and radiographic analysis of an optimized rh-BMP-2 formulation as an autograft replacement in posterolateral lumbar spine arthrodesis. J Bone Joint Surg Am. 2009. 91: 1377-86
12. Haid RW, Branch CL, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004. 4: 527-38
13. Hussain A, Erdek M. Interventional pain management for failed back surgery syndrome. Pain Pract. 2014. 14: 64-78
14. Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: A CT analysis. Spine. 2007. 32: 2885-90
15. Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J.editors. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicenter randomized controlled trial in patients with failed back surgery syndrome. Pain. 2007. 132: 179-88
16. Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J. The effects of spinal cord stimulation in neuropathic pain are sustained: A 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008. 63: 762-70
17. Linderoth B, Fedorcsak I, Meyerson BA. Peripheral vasodilatation after spinal cord stimulation: Animal studies of putative effector mechanisms. Neurosurgery. 1991. 28: 187-95
18. Linderoth B, Gunasekera L, Meyerson BA. Effects of sympathectomy on skin and muscle microcirculation during dorsal column stimulation: Animal studies. Neurosurgery. 1991. 29: 874-9
19. Linderoth B, Herregodts P, Meyerson BA. Sympathetic mediation of peripheral vasodilation induced by spinal cord stimulation: Animal studies of the role of cholinergic and adrenergic receptor subtypes. Neurosurgery. 1994. 35: 711-9
20. Melzack R, Wall PD. Pain mechanisms: A new theory. Science. 1965. 150: 971-9
21. North RB, Kidd D, Shipley J, Taylor RS. Spinal cord stimulation versus reoperation for failed back surgery syndrome: A cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery. 2007. 61: 361-9
22. Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine. 2010. 35: 1794-800
23. Raphael JH, Muntagi H, kapur S. Spinal cord stimulation and its anesthetic implications. Contin Ed Anaest Crit care Pain. 2009. 9: 78-81
24. Slipman CW, Shin CH, Patel RK, Isaac Z, Huston CW, Lipetz JS. Etiologies of failed back surgery syndrome. Pain Med. 2002. 3: 200-14
25. Tannoury CA, An HS. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014. 14: 552-9
26. Urist MR, Mikulski A, Lietze A. Solubilized and insolubilized bone morphogenetic protein. Proc Natl Acad Sci. 1979. 76: 1828-32
27. Urist MR. Bone: Formation by autoinduction. Clin Orthop Relat Res. 2002. p. 4-10
28. cited January 4, 2016; updated September 5, 2013. Available at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm083423.htm.
29. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: A potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008. 8: 1011-8
30. Zlotolow DA, Vaccaro AR, Salamon ML, Albert TJ. The role of human bone morphogenetic proteins in spinal fusion. J Am Acad Orthop Surg. 2000. 8: 3-9