- Department of Neurosurgery, College of Medicine, University of Florida, Gainesville, Florida, United States.
- Department of Neurosurgery, Jackson Memorial Hospital, University of Miami Miller School of Medicine, Miami, Florida, United States.
- Department of Neurosurgery, University of Miami Miller School of Medicine, Miami, Florida, United States.
Correspondence Address:
Jasmina Kovacevic, Department of Neurosurgery, College of Medicine, University of Florida, Gainesville, Florida, United States.
DOI:10.25259/SNI_357_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jasmina Kovacevic1, Michael Alexander Silva2, Henry Chang3, Mynor Josue Mendez Valdez3, Ian Ramsay3, Uche C. Ezeh3, Andres M. Corona3, Ahmed Abdelsalam3, Robert M. Starke3. Spontaneous closure of a superior sagittal sinus dural arteriovenous fistula with an extensive angioarchitectural network: A case report and systematic review of the literature. 07-Jul-2023;14:239
How to cite this URL: Jasmina Kovacevic1, Michael Alexander Silva2, Henry Chang3, Mynor Josue Mendez Valdez3, Ian Ramsay3, Uche C. Ezeh3, Andres M. Corona3, Ahmed Abdelsalam3, Robert M. Starke3. Spontaneous closure of a superior sagittal sinus dural arteriovenous fistula with an extensive angioarchitectural network: A case report and systematic review of the literature. 07-Jul-2023;14:239. Available from: https://surgicalneurologyint.com/surgicalint-articles/12391/
Abstract
Background: Intracranial dural arteriovenous fistulas (DAVFs) have been documented to occasionally spontaneously regress. However, the mechanism responsible for this occurrence remains speculative.
Methods: We present a case of a Borden II – Cognard IIa+b DAVF involving the superior sagittal sinus (SSS) with bilateral external carotid artery supply that regressed spontaneously. A systematic literature review was conducted to explore the current theories explaining the spontaneous regression of DAVFs according to Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines.
Results: A total of 26 studies and 54 cases were included in our results. Of the included cases, 57.14% of cases were Borden I, 16.33% were Borden II, and 26.53% were Borden III. Ruptured status or intracranial hemorrhage was documented in 24.1% of all cases, the majority of which (69.2%) were in cases with aggressive lesions (Borden II or greater). The most commonly involved location was the transverse sinus (38.89% of cases, n = 21), and the SSS was only involved in 12.96% of all cases. 50% of included cases proposed a mechanism responsible for spontaneous regression. The most frequently proposed mechanisms were thrombosis of the involved sinus/chronic inflammatory changes or direct endothelial injury, endoluminal stasis, and thrombogenic effects of contrast medium during angiography. We present the case of a 54-year-old woman with an aggressive ruptured DAVF that likely developed following a pediatric traumatic brain injury that was left untreated before she presented to our institution after significant delay. Her DAVF regressed on repeat angiography before neurovascular intervention without a clear identifying mechanism as proposed by the current literature.
Conclusion: Our results suggest that spontaneous regression is not necessarily associated with lower risk DAVFs. The present case offers a unique long-term insight into the natural history of an aggressive ruptured DAVF of the SSS that regressed without intervention. Further research into the natural history of DAVFs will be helpful in deducing key factors leading to spontaneous regression.
Keywords: Angiography, Dural arteriovenous fistula, Intracerebral hemorrhage, Spontaneous closure, Superior sagittal sinus
INTRODUCTION
Intracranial dural arteriovenous fistulas (DAVFs) are vascular malformations that form within the dura involving abnormal connections between dural or meningeal arteries and dural, arachnoid, or cortical veins.[
Spontaneous regression of DAVFs was classically thought to be associated with less aggressive lesions (Borden I, Cognard IIa or lower), yet recent studies have highlighted that more aggressive DAVFs may regress as well.[
SYSTEMATIC REVIEW METHODOLOGY
We conducted a systematic review of the literature regarding the spontaneous resolution of DAVFs in accordance with the guidelines set forth by the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement.[
RESULTS
Case report
A 54-year-old woman with a history of motor vehicle accident at the age of 4 years, complicated by traumatic brain injury requiring craniectomy and evacuation of hematoma, presented to an outside institute with an acute episode of vomiting associated with elevation in blood pressure, right-sided weakness, intermittent diplopia, and seizure. The patient was admitted to the intensive care unit and then discharged after a week but suffered from a repeat episode of seizure 2 months later with new onset receptive and expressive aphasia.
A brain magnetic resonance imaging (MRI) revealed hyperintensity in the left temporal and parietal lobes, which was thought to be caused by congestion secondary to venous hypertension. Blood products were also noted on the left parietal lobe with gyral enhancement, presumably from prolonged venous congestion with cortical vein/ dural thrombosis or hemorrhage in the past. Notably, the superior sagittal sinus (SSS) filled irregularly and was suggestive of a thrombotic process.
A cerebral angiogram was then performed which revealed a DAVF (Borden II – Cognard IIa+b) involving the middle to posterior thirds of the SSS supplied by multiple arterial sources [
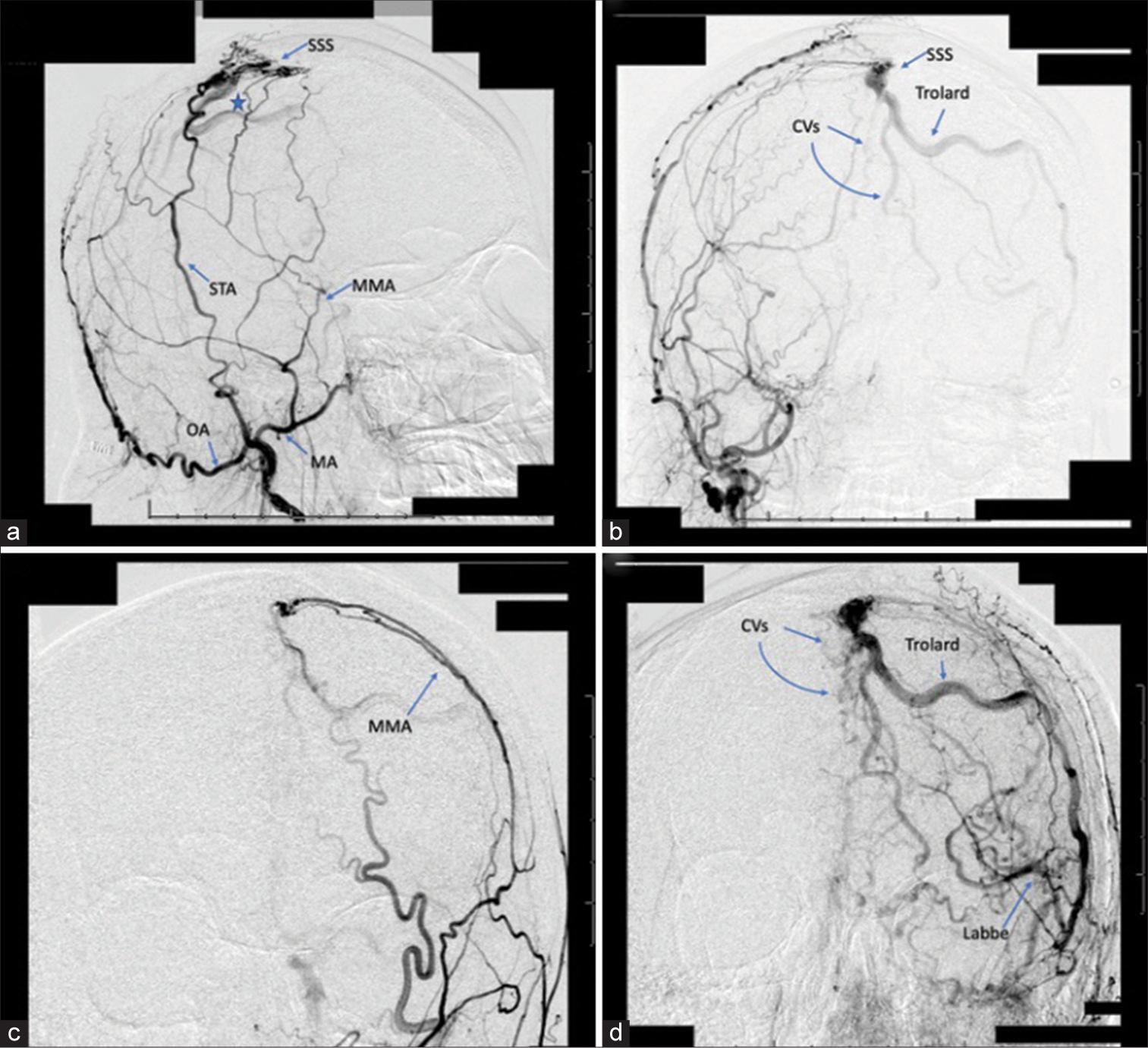
Figure 1:
A 54-year-old woman with a dural arteriovenous fistula (DAVF) involving the superior sagittal sinus (SSS). (a) Right external carotid artery (ECA) angiogram, arterial phase, demonstrating a long segment DAVF (Borden II – Cognard IIa+b) involving the middle to posterior thirds of the SSS with supply from the right superficial temporal artery and right middle meningeal artery (MMA) into the middle third of the SSS (star). (b) Right ECA angiogram, late arterial phase, demonstrating reflux into cortical veins (CVs) and occlusion of the SSS. (c) Left common carotid injection, arterial phase, demonstrating a fistulous connection between the left MMA and the middle third of the SSS. (d) Left common carotid injection, venous phase, revealing reflux into CVs and the left vein of Trolard. Labbe: Inferior anastomotic vein of Labbe, MMA: Middle meningeal artery, OA: Occipital artery, STA: Superficial temporal artery.
Brain computed tomography (CT) 1 month later demonstrated chronic infarct of the left parietal lobe with cortical laminar necrosis in both the left parietal and temporal lobes. These results remained stable at 2-month and 6-month repeat brain CTs. The patient was started on dabigatran for her venous sinus thrombosis based on decision from the patient’s outside neurologist and interventionalist.
When the patient presented to our institute 1 year later never having received treatment for the DAVF, she had residual weakness on the right side, mild gait imbalance, and short-term memory deficit. Given the complexity of the DAVF and the patient’s age, alongside history of stroke/hemorrhage, the decision was made to proceed with a repeat cerebral angiogram to evaluate for changes in the angioarchitecture of the DAVF in an effort to plan for neurovascular intervention. However, on repeat angiogram, there was no evidence of fistula noted; the DAVF had self-obliterated without treatment. The SSS remained occluded, and drainage was noted through extensive dilatated cortical veins as collaterals [
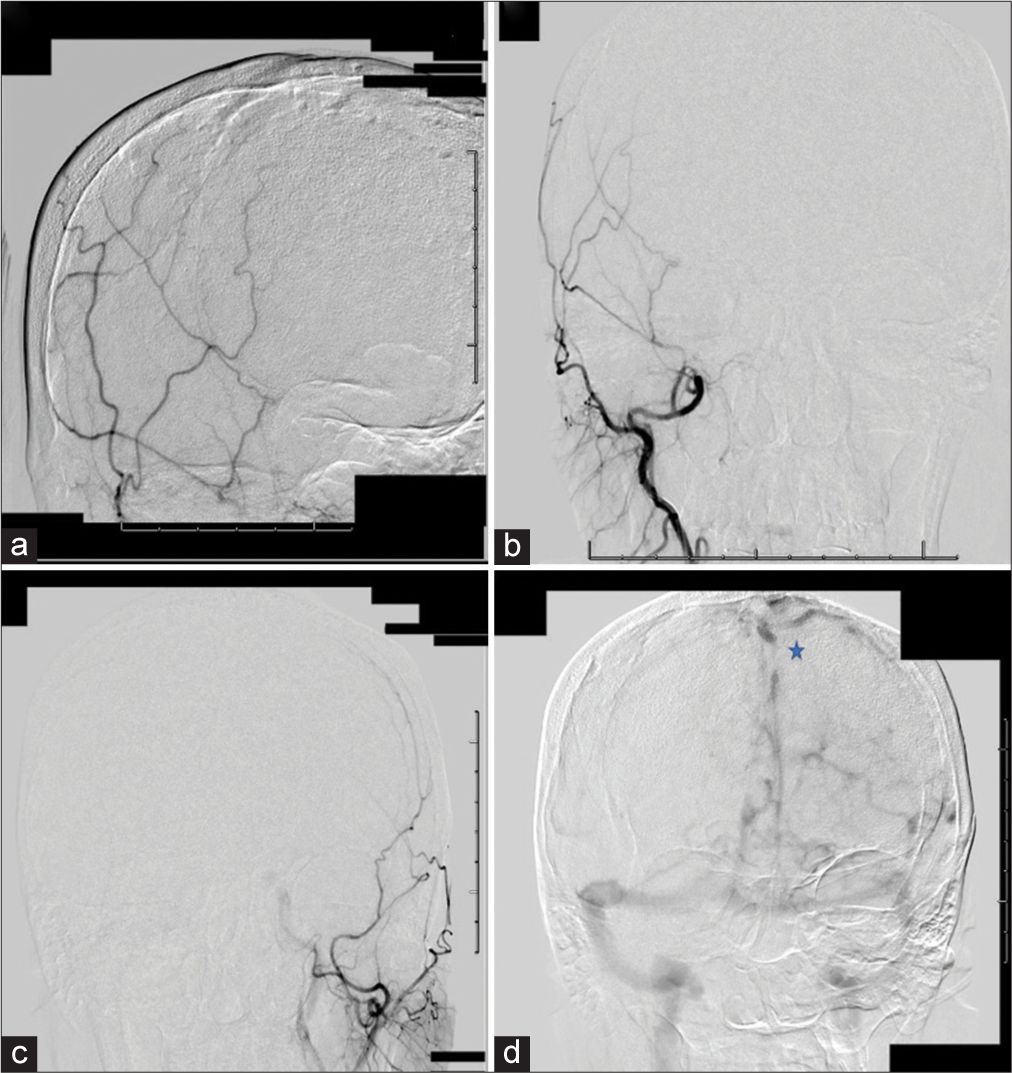
Figure 2:
Angiogram performed 1 year later. Right external carotid artery (ECA) angiogram, lateral (a) and anterior-posterior (b) views, demonstrating patent filling of the right ECA and its branches. No evidence of early venous drainage to suggest dural arteriovenous fistula (DAVF). (c) Common carotid injection, arterial phase, revealing normal filling and no evidence of fistula. (d) Left internal carotid injection, venous phase, demonstrating no evidence of DAVF. Superior sagittal sinus thrombosis and venous engorgement of cortical veins (star) are observed.
REVIEW OF THE LITERATURE
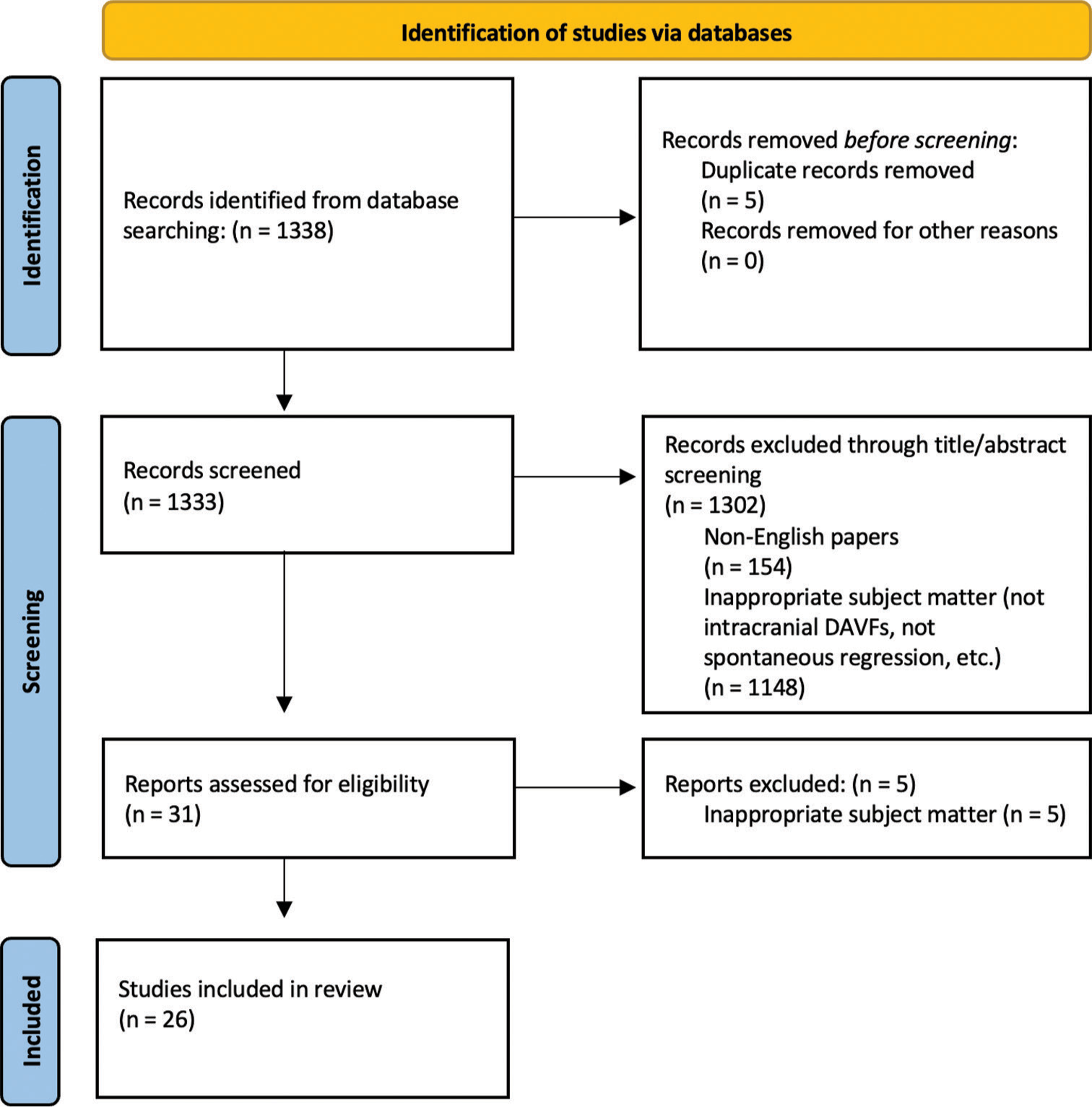
A systematic review was conducted to examine other cases of intracranial DAVFs with spontaneous resolution in the scientific literature. Our search querying PubMed yielded 1338 journal articles. Of our results, we included 26 articles detailing 54 cases of intracranial DAVFs spontaneously regressing [
Based on Borden classification, 57.14% of cases were Borden I, 16.33% were Borden II, and 26.53% were Borden III; 34.48% of DAVFs were Cognard I, 10.34% were Cognard IIa, 10.34% were Cognard IIb, 17.24% were Cognard IIa+b, 20.69% were Cognard III, and 6.90% were Cognard IV. Neither patient age nor sex were found to be associated with Borden (P = 0.754, age; P = 0.818, sex; ordinal logistic regression) or Cognard distributions (P = 0.554, age; P = 0.7, sex; ordinal logistic regression). The most frequently involved sinus was the transverse sinus (38.89% of cases, n = 21); the SSS was involved in 7 cases (12.96%). Intracranial hemorrhage was observed at the time of DAVF diagnosis in 13 cases (24.1%). Neither involvement of the SSS nor rupture status had significant associations with lapse between DAVF diagnosis and spontaneous regression (P = 0.626, P = 0.127, respectively, linear regression). The relationship between hemorrhagic or ruptured presentation and higher-risk lesions (Borden II or greater, Cognard IIb or greater) had a trend toward significance (P = 0.063, logistic regression).
Of the included cases that proposed a reason for spontaneous regression, the most commonly cited factors were thrombosis of involved sinus/chronic inflammatory changes and fibrosis (24.07% of reported cases, n = 13) and arteriography/attempted neurovascular intervention-associated reasons (18.52% of reported cases, n = 10). Of all included cases, 50% (n = 27) identified no definitive factors responsible for spontaneous regression of DAVF. Of cases that reported sinus status (patent vs. occluded) at the time of DAVF diagnosis and the time of regression (n = 24), 41.67% of cases (n = 10) reported sinus patency throughout the period when DAVF regression occurred, 29.17% (n = 7) reported persistent sinus occlusion, 25% of cases (n = 6) demonstrated recanalization of involved sinus, and 4.17% (n = 1) documented new onset sinus occlusion at the time of DAVF regression.
DISCUSSION
From our literature review, the cases documenting the spontaneous regression of intracranial DAVFs were nearly balanced between low-risk lesions (57.14% Borden I, 44.82% Cognard IIa or lower) and aggressive high-risk lesions (42.58% Borden II or greater and 55.18% Cognard IIb or greater). This supports the notion that lower risk DAVFs do not necessarily portend to higher rates of spontaneous regression.[
Several mechanisms underlying the spontaneous regression of DAVFs have been proposed in the literature. Magidson and Weinberg initially proposed the involvement of sinus thrombosis in the mechanism for the spontaneous regression of a Borden I DAVF involving the transverse sinus in 1976.[
A report by Kataoka and Taneda[
Another commonly cited mechanism for spontaneous regression involves attempted neurovascular intervention or the impact of angiography and associated contrast medium. From our systematic review, the two main suggested mechanisms involve either direct endothelial injury and endoluminal stasis induced by neurosurgical intervention or thrombogenic effects of contrast medium during imaging.[
The thrombogenic effects of contrast media have also been documented and emphasized in the cases of DAVFs with unique feeding arteries or those with a small nidus and single draining vein,[
Compression of DAVF feeders or shunts by a hematoma, hematoma-associated mass effect, or edema has also been proposed as a possible mechanism for spontaneous DAVF occlusion.[
An interesting case was reported by van Beijnum et al.[
In the cases that involved the SSS (n = 7), the most often cited mechanism remained venous sinus or arteriosinus shunt thrombosis (n = 4), followed by angiography or contrast media-related thrombogenic effects (n = 2). Of note, two of those cases speculated that angioarchitecture played a contributing role in the spontaneous occlusion of their DAVFs: Intravascular turbulence from fistula tortuosity[
In the present case, our patient suffered a traumatic brain injury from a motor vehicle accident as a child, requiring a craniectomy and hematoma evacuation that likely led to the occlusion of the SSS. The resultant venous congestion/ hypertension and infarct of the parietal lobe likely promoted DAVF angiogenesis and development. Subsequently, the chronic venous hypertension likely contributed to maintaining the patency of the DAVF. It is also possible that the venous sinus hypertension ultimately played a role in compressing shunt connections, leading to spontaneous regression; however, this mechanism is less probable given our patient’s extensive and bilateral angioarchitecture network. Therefore, if venous hypertension was responsible for the maintenance of the DAVF in our patient, we considered the possibility that the initiation of dabigatran and subsequent resolution of the SSS occlusion could explain the regression of the DAVF. However, the SSS remained occluded at repeat angiography, suggesting that recanalization was not the mechanism behind our patient’s DAVF regression. It is possible that the thrombus occluding the SSS gradually fibrosed and led to chronic inflammation and stenosis, leading to the eventual occlusion of the DAVF; the patient’s hemorrhagic presentation – with resultant inflammatory and hemodynamic changes – alongside prior angiogram 1 year before presenting to our institute could have also had prothrombotic effects that further acutely contributed to the spontaneous occlusion of the DAVF.
Our case documents a DAVF with an extensive network, involving supply from the internal carotid (anterior falx/ falcine artery), vertebral artery (posterior inferior cerebellar artery), and bilateral external carotids (MMA, STA, occipital artery), that spontaneously regressed without a clear identifying mechanism as proposed by the current literature. One other SSS DAVF with bilateral supply was identified in our systematic review; however, the patient had a single draining vein that was speculated to have had a contributing role in the eventual regression of their DAVF.[
Several limitations of our study should be acknowledged. First, our review included predominantly case reports and case series due to the low documented incidence of DAVF with spontaneous regression. Furthermore, high-risk DAVFs are generally recommended for intervention and therefore the long-term insight into their natural history is limited in the literature. Second, the limited number of reported DAVF with spontaneous regression and the lack of consistent and detailed reporting among the included studies (e.g., sinus patency before and after spontaneous resolution, and proposed mechanism explaining spontaneous resolution) limited the power of our statistical analysis. Third, the inherent limitations of a systematic review based on published articles in the literature include publication bias.
CONCLUSION
In our systematic review of 26 articles and 54 cases of spontaneous regressions of DAVFs, 57% were Borden I and 43% were Borden II or greater. DAVFs that involved the SSS were all Borden II or greater and 71% of them presented with hemorrhage. The most often proposed mechanisms for spontaneous regression – among the total cohort and the subset of cases involving the SSS – involved sinus thrombosis and chronic inflammatory changes or fibrosis, followed by angiography/attempted neurovascular intervention associated factors. However, there is no consensus in the literature regarding the mechanisms responsible for the natural regression of DAVFs, with 50% of our included cases without an identifiable mechanism proposed. Our report on a 54-year-old woman provides a distinctive and extended perspective on the natural progression of a high-risk ruptured DAVF that remained untreated for a considerable duration. The regression of her DAVF occurred without a clear identifying mechanism as proposed by the current literature. DAVFs are dynamic diseases, and further research into their natural history will be needed to deduce key factors in their spontaneous regressions.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflict of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Agnoletto GJ, Hoover JM, Monteiro A, Hanel RA. Catch me if you can: Disappearing and reappearing posterior fossa dural arteriovenous malformation. BMJ Case Rep. 2019. 12: e229437
2. Ahn JY, Lee BH, Cho YJ, Joo JY, Lee KS. Dural arteriovenous fistula associated with meningioma: Spontaneous disappearance after tumor removal. Neurol Med Chir (Tokyo). 2003. 43: 308-11
3. Al-Afif S, Nakamura M, Gotz F, Krauss JK. Spontaneous closure of a dural arteriovenous fistula. BMJ Case Rep. 2014. 2014: bcr2014011255
4. Alkhaibary A, Alnefaie N, Alharbi A, Alammar H, Arishy AM, Alshaya W. Intracranial dural arteriovenous fistula: A comprehensive review of the history, management, and future prospective. Acta Neurol Belg. 2022. 123: 359-66
5. Basheer N, Kasliwal MK, Gaikwad S, Sharma BS. Spontaneous closure of dural arterio-venous fistula. Neurol India. 2008. 56: 207
6. Beniwal M, Saini J, Somanna S, Deepesh A, Rao KV, Vazhayil V. Spontaneous closure of dural arteriovenous fistula; a visual specter. Neurol India. 2019. 67: 1376-9
7. Blomquist MH, Barr JD, Hurst RW. Isolated unilateral hypoglossal neuropathy caused by dural arteriovenous fistula. AJNR Am J Neuroradiol. 1998. 19: 951-3
8. Chaudhary MY, Sachdev VP, Cho SH, Weitzner I, Puljic S, Huang YP. Dural arteriovenous malformation of the major venous sinuses: An acquired lesion. AJNR Am J Neuroradiol. 1982. 3: 13-9
9. Chen PM, Chen MM, McDonald M, McGehrin K, Steinberg J, Handwerker J. Cranial dural arteriovenous fistula. Stroke. 2018. 49: e332-4
10. Clarençon F, Biondi A, Sourour NA, Di Maria F, Iosif C, Nouet A. Spontaneous closure of intracranial dural arteriovenous fistulas: A report of 3 cases. Clin Neurol Neurosurg. 2013. 115: 971-5
11. Endo S, Koshu K, Suzuki J. Spontaneous regression of posterior fossa dural arteriovenous malformation. J Neurosurg. 1979. 51: 715-7
12. Hansen JH, Sogaard I. Spontaneous regression of an extra-and intracranial arteriovenous malformation. Case report. J Neurosurg. 1976. 45: 338-41
13. Iampreechakul P, Wangtanaphat K, Lertbutsayanukul P, Wattanasen Y, Siriwimonmas S. Spontaneous closure of a cavernous sinus dural arteriovenous fistula with spinal perimedullary drainage (cognard V) during attempted transvenous embolization. Asian J Neurosurg. 2019. 14: 1268-74
14. Kannath SK, Rajan JE, Mukherjee A, Sarma PS. Factors predicting spontaneous thrombosis of aggressive cranial dural arteriovenous fistulas. World Neurosurg. 2017. 103: 821-8.e2
15. Kashiwagi N, Miyazaki K, Takahashi H, Tsuji K, Fujiwara M, Arisawa A. Spontaneous closure of non-cavernous sinus dural arteriovenous fistulas: A case series and systematic review of the literature. J Neuroradiol. 2022. 49: 94-100
16. Kataoka K, Taneda M. Angiographic disappearance of multiple dural arteriovenous malformations. Case report. J Neurosurg. 1984. 60: 1275-8
17. Kim DJ, TerBrugge K, Krings T, Willinsky R, Wallace C. Spontaneous angiographic conversion of intracranial dural arteriovenous shunt: Long-term follow-up in nontreated patients. Stroke. 2010. 41: 1489-94
18. Kutluk K, Schumacher M, Mironov A. The role of sinus thrombosis in occipital dural arteriovenous malformations--development and spontaneous closure. Neurochirurgia (Stuttg). 1991. 34: 144-7
19. Landman JA, Braun IF. Spontaneous closure of a dural arteriovenous fistula associated with acute hearing loss. AJNR Am J Neuroradiol. 1985. 6: 448-9
20. Luciani A, Houdart E, Mounayer C, Maurice JP, Merland JJ. Spontaneous closure of dural arteriovenous fistulas: Report of three cases and review of the literature. AJNR Am J Neuroradiol. 2001. 22: 992-6
21. Magidson MA, Weinberg PE. Spontaneous closure of a dural arteriovenous malformation. Surg Neurol. 1976. 6: 107-10
22. Manabe S, Satoh K, Matsubara S, Satomi J, Hanaoka M, Nagahiro S. Characteristics, diagnosis and treatment of hypoglossal canal dural arteriovenous fistula: Report of nine cases. Neuroradiology. 2008. 50: 715-21
23. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009. 6: e1000097
24. Olutola PS, Eliam M, Molot M, Talalla A. Spontaneous regression of a dural arteriovenous malformation. Neurosurgery. 1983. 12: 687-90
25. Pritz MB, Pribram HF. Spontaneous closure of a high-risk dural arteriovenous malformation of the transverse sinus. Surg Neurol. 1991. 36: 226-8
26. Reul J, Thron A, Laborde G, Bruckmann H. Dural arteriovenous malformations at the base of the anterior cranial fossa: Report of nine cases. Neuroradiology. 1993. 35: 388-93
27. Saito A, Furuno Y, Nishimura S, Kamiyama H, Nishijima M. Spontaneous closure of transverse sinus dural arteriovenous fistula: Case report. Neurol Med Chir (Tokyo). 2008. 48: 564-8
28. Tsuji K, Nakagawa N, Fukawa N, Kato A. Spontaneous closure of a dural arteriovenous fistula immediately after cerebral angiography using a gadolinium contrast agent. J Stroke Cerebrovasc Dis. 2014. 23: e449-52
29. Van Beijnum J, Klijn CJ, Lo TH, van der Zwan A, Kappelle LJ. Spontaneous obliteration of a dural arteriovenous fistula after treatment of polycythemia in a patient with factor V Leiden mutation: Case report. J Neurol. 2010. 257: 1573-5
30. Voormolen V, Geens K, Van Den Hauwe L, Parizel PM. Spontaneous closure of cerebral dural arteriovenous fistulas with direct cortical venous drainage. A case report. Interv Neuroradiol. 2009. 15: 359-62
31. Warren DJ, Craven I, Romanowski CA, Coley SC. Spontaneous closure of a Type 2a dural arteriovenous fistula following late recanalization of the occluded sinus. Interv Neuroradiol. 2010. 16: 282-5