- Department of Neurosurgery, Neurological Institute of Thailand, Bangkok, Thailand
- Department of Neuroradiology, Neurological Institute of Thailand, Bangkok, Thailand
- Department of Radiology, Bumrungrad International Hospital, Bangkok, Thailand.
Correspondence Address:
Prasert Iampreechakul, Department of Neurourgery, Neurological Institute of Thailand, 312 Rachawithi Road, Khwaeng Thung Phaya Thai, Bangkok, 10400, Thailand.
DOI:10.25259/SNI_594_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Prasert Iampreechakul1, Korrapakc Wangtanaphat1, Songpol Chuntaroj2, Yodkhwan Wattanasen2, Sunisa Hangsapruek2, Punjama Lertbutsayanukul2, Somkiet Siriwimonmas3. Spontaneous complete regression of malignant cavernous sinus dural arteriovenous fistula following partial transarterial embolization with liquid embolic material: Report of two cases. 01-Sep-2023;14:307
How to cite this URL: Prasert Iampreechakul1, Korrapakc Wangtanaphat1, Songpol Chuntaroj2, Yodkhwan Wattanasen2, Sunisa Hangsapruek2, Punjama Lertbutsayanukul2, Somkiet Siriwimonmas3. Spontaneous complete regression of malignant cavernous sinus dural arteriovenous fistula following partial transarterial embolization with liquid embolic material: Report of two cases. 01-Sep-2023;14:307. Available from: https://surgicalneurologyint.com/surgicalint-articles/12524/
Abstract
Background: Spontaneous complete regression of malignant cavernous sinus dural arteriovenous fistulas (CSDAVFs) following partial transarterial embolization is an extremely uncommon phenomenon. The mechanism responsible for this condition remains unclear.
Case Description: The authors describe two cases of malignant CSDAVFs (Cognard IIb and V) treated by partial transarterial embolization with liquid embolic agents after unsuccessful transvenous embolization through various routes. Follow-up cerebral angiography in these cases confirmed complete resolution of the fistulas.
Conclusion: In our two patients harboring low-flow CSDAVFs with preexisting thrombosis of the cavernous sinus (CS), it is possible that some portions of the liquid embolic materials could migrate into the fistulas, inducing thrombosis within the CS.
Keywords: Cavernous sinus dural arteriovenous fistula, Intracranial dural arteriovenous fistula, Spontaneous closure, Spontaneous regression, Transarterial embolization
INTRODUCTION
Cavernous sinus dural arteriovenous fistulas (CSDAVFs) are abnormal communications between dural arteries and the cavernous sinus (CS). Spontaneous CSDAVFs, low flow, and low pressure, commonly appear in middle-aged females, and symptoms of the patients usually relate to venous drainage patterns.[
Based on the pattern of venous drainage by Cognard et al.,[
At present, a transfemoral transvenous approach through the ipsilateral inferior petrosal sinus (IPS) is recommended as the first-line treatment of CSDAVFs.[
CASE DESCRIPTION
Case 1
A 32-year-old woman noticed headache with proptosis and redness of the right eye for 1 month. There was no history of trauma. She went to the local hospital and received contrast-enhanced cranial computed tomography (CT) scan that showed abnormal bulging of the right CS with dilated right superior ophthalmic vein (SOV). The provisional diagnosis was the right CSDAVF. She was transferred to our institute for further investigation and treatment. Cerebral angiography revealed the right CSDAVF supplied from the right artery of the foramen rotundum, accessory meningeal artery, middle meningeal artery (MMA), and dural branches of the bilateral cavernous segment of internal carotid arteries (ICAs) with drainage into the right mildly dilated SOV and superior petrosal sinus (SPS) with subsequently draining into the right sigmoid sinus. In addition, drainage was through the right lateral medullary veins into the dilated spinal perimedullary veins, corresponding with a classification of a Cognard type V DAVF [
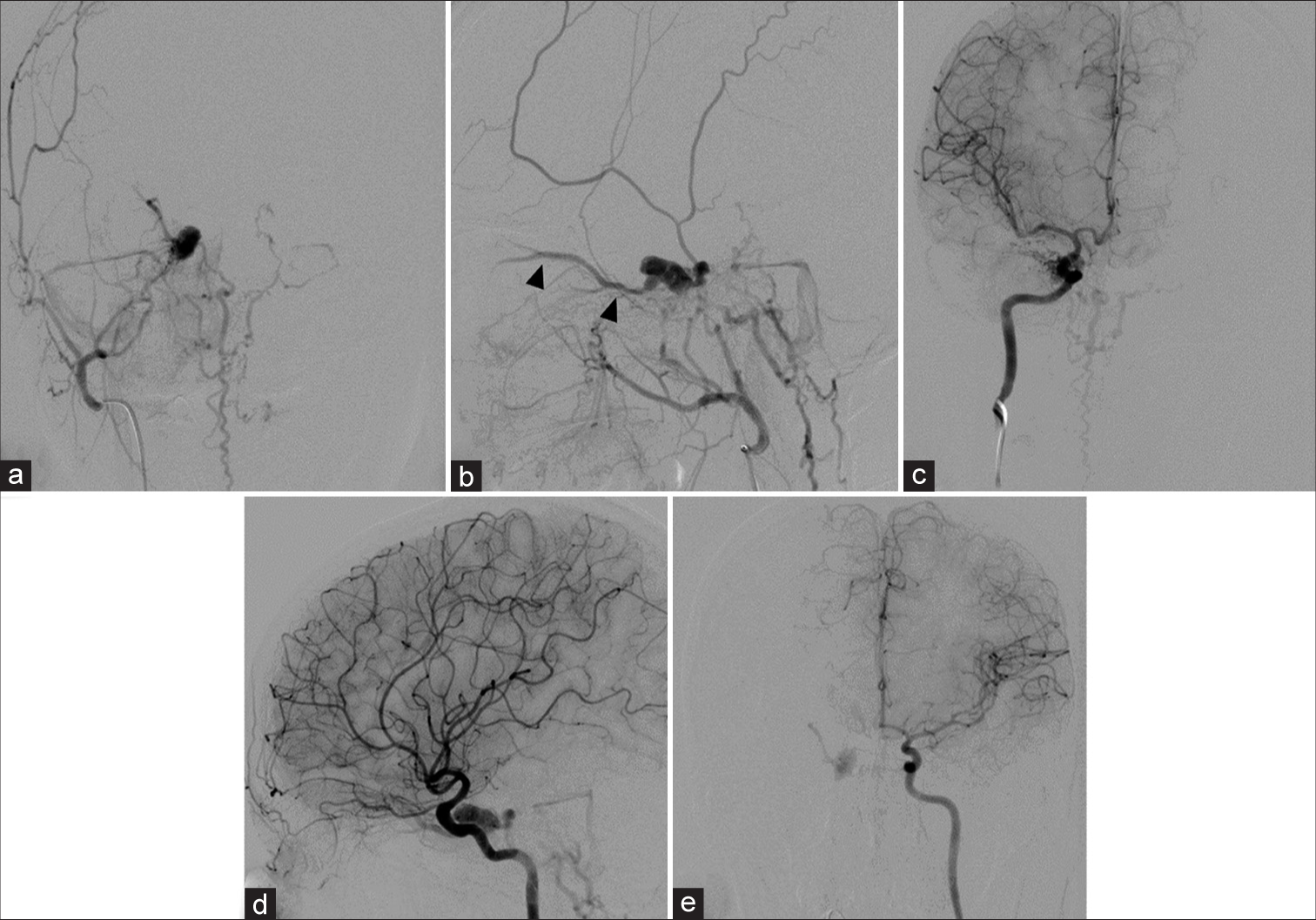
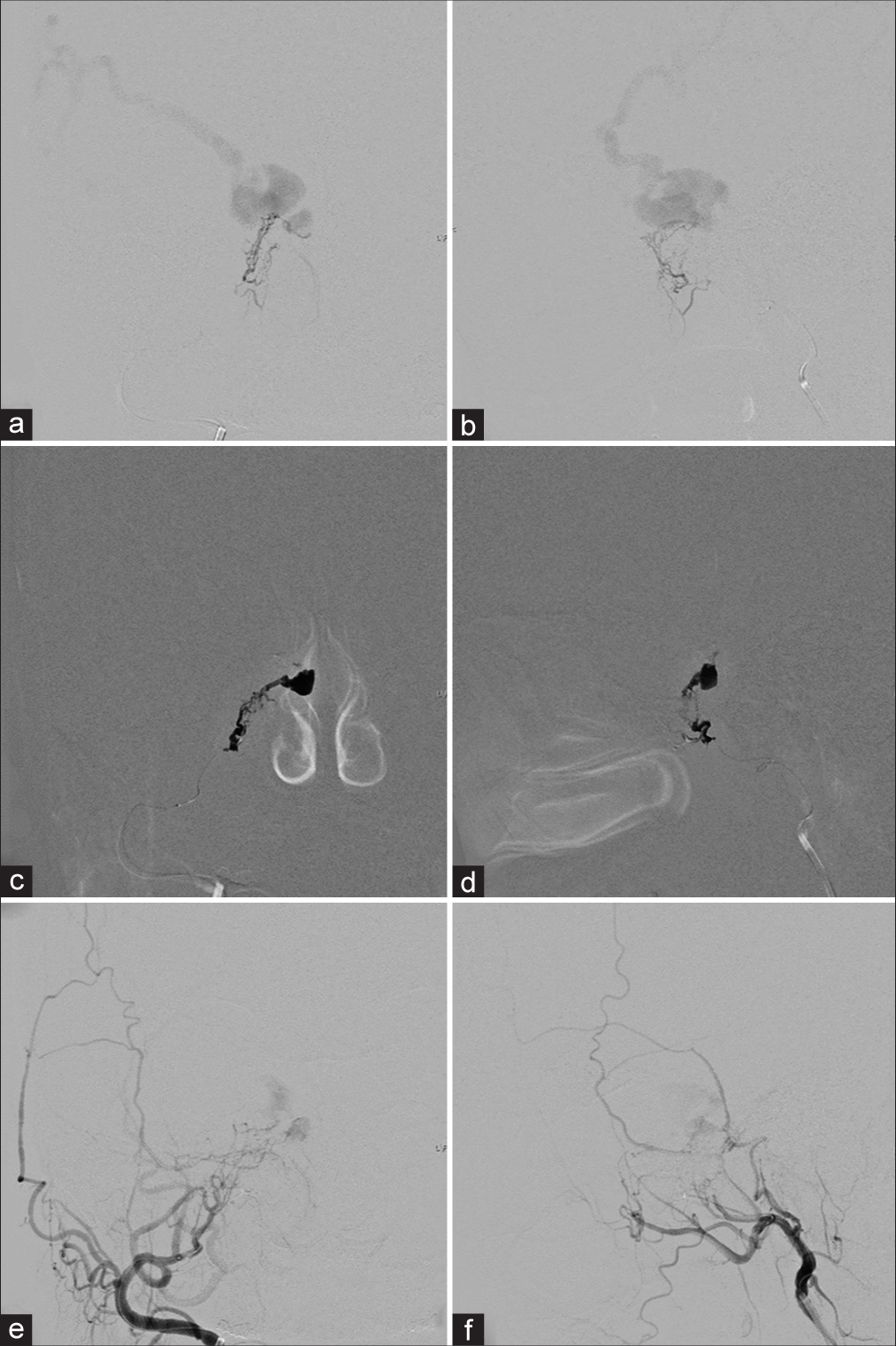
Figure 1:
(a) Anteroposterior (AP) and (b) lateral views of the right external carotid artery injections show the right cavernous sinus dural arteriovenous fistula supplied from the right artery of the foramen rotundum, accessory meningeal artery, middle meningeal artery with drainage into the right superior ophthalmic vein (SOV), superior petrosal sinus and spinal medullary veins. There is partial filling of the right SOV (arrowheads) incompatible with the patient’s ocular symptoms, probably representing pre-existing thrombosis. (c) AP and (d) lateral views of the right internal carotid artery (ICA) injections reveal the vascular supply from meningohypophyseal trunk (MHT). (e) AP view of the left ICA injection demonstrates the clival branch from the left MHT supplying the fistula.
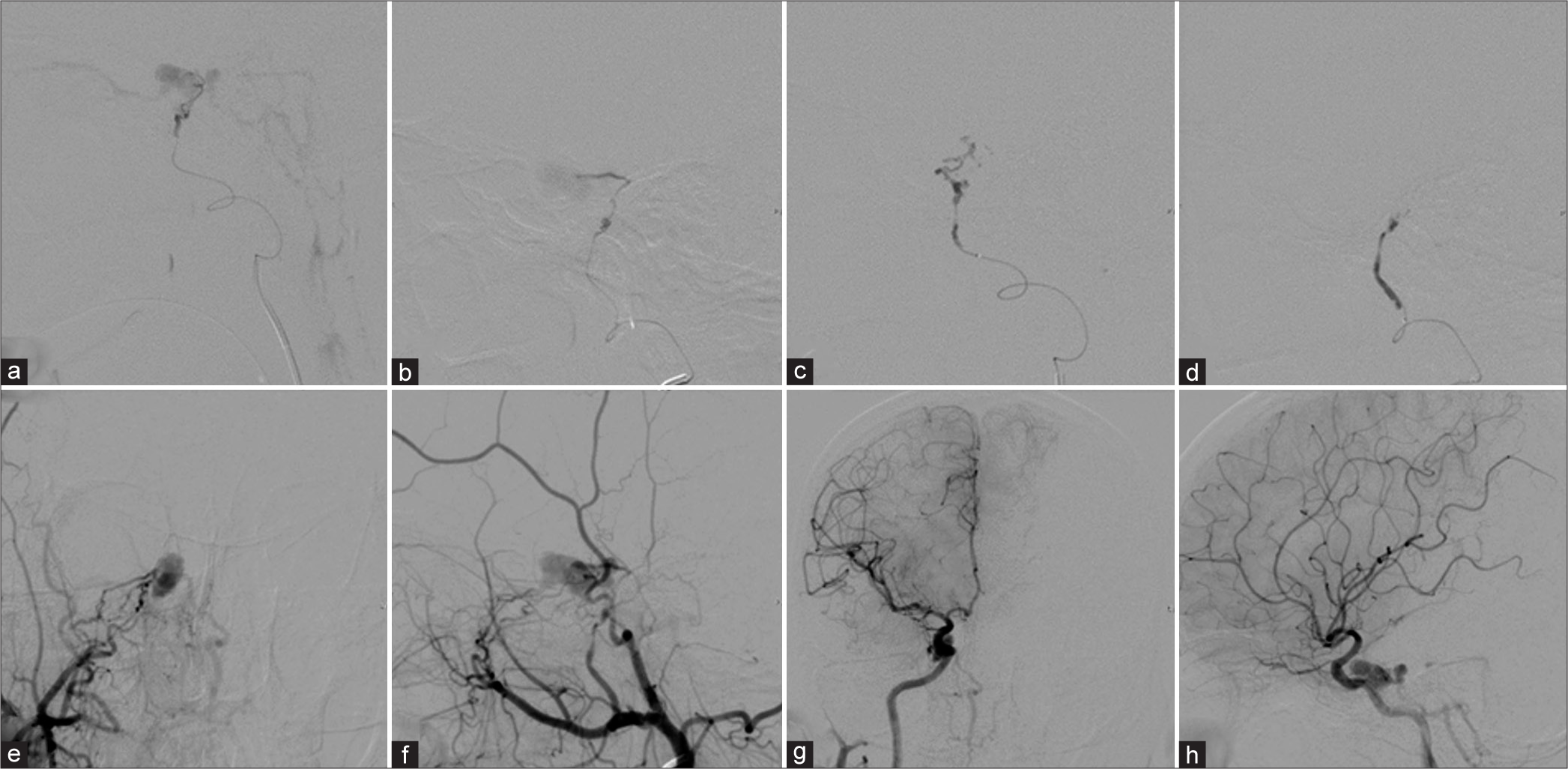
Figure 2:
Lateral views of the right (a) accessory meningeal artery and (b) middle meningeal artery injections before embolization. During glue embolization from the right (c) accessory meningeal artery and (d) middle meningeal artery. (e) Anteroposterior (AP) and (f) lateral views of the right external carotid artery and (g) AP and (h) lateral views of the right internal carotid artery injections demonstrate incomplete obliteration of the fistula.
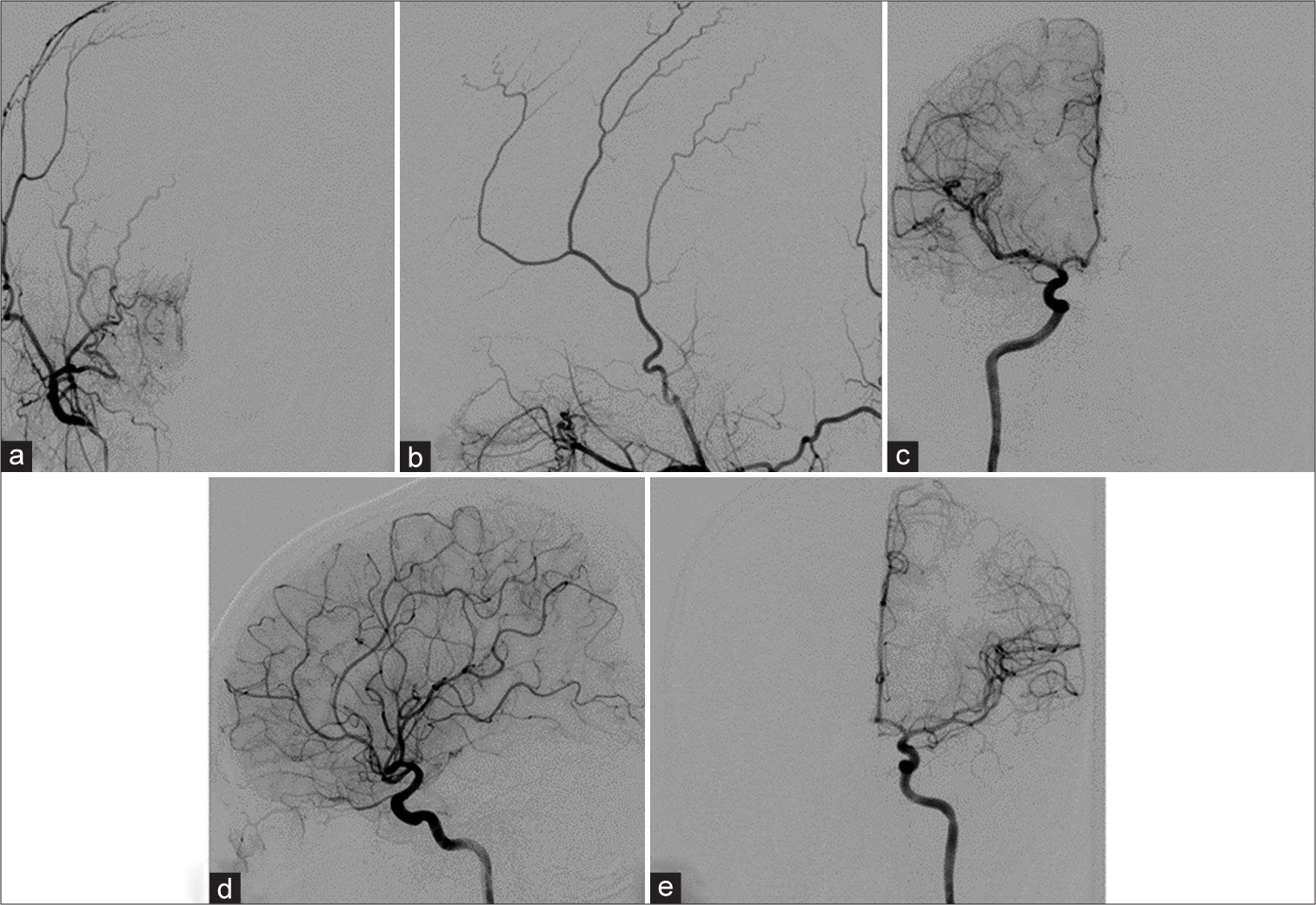
Figure 3:
Cerebral angiography obtained 6 months after embolization. (a) Anteroposterior (AP) and (b) lateral views of the right external carotid artery, (c) AP and (d) lateral views of the right internal carotid artery (ICA), and (e) AP view of the left ICA injections confirms complete obliteration of the right cavernous sinus dural arteriovenous fistula.
Case 2
A 52-year-old woman went to the local hospital due to proptosis and redness of the right eye for 4 days. One month earlier, she noticed tinnitus in her right ear. There was no history of trauma. Contrast-enhanced CT scan of the brain revealed abnormal bulging of the right CS with dilated right SOV. The patient was diagnosed with the right CSDAVF and sent to our institute. Unfortunately, she could not come to our institute at that moment due to the COVID-19 pandemic. Two months later, she developed a mild headache and progression of her eye symptoms for 1 week. Later, her ocular symptoms and tinnitus disappeared totally. Six months later, the patient came to our institute for further investigation and treatment. Cerebral angiography showed the right CSDAVF fed by the right artery of the foramen rotundum, accessory meningeal artery, MMA, and dural branches of the bilateral cavernous segment of ICAs with drainage into the right dilated superficial middle cerebral and Labbé veins, corresponding with a classification of a Cognard type IIb DAVF [
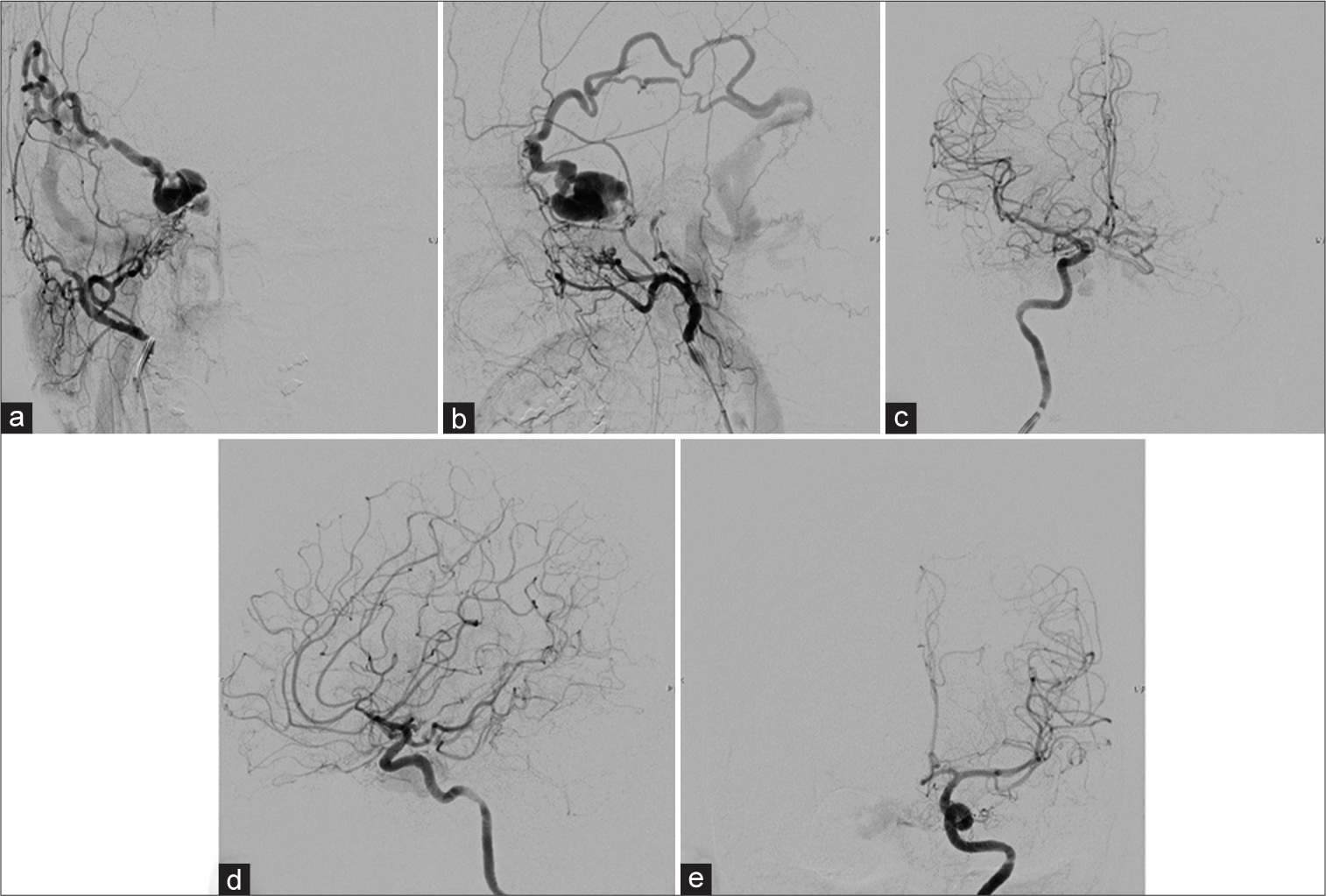
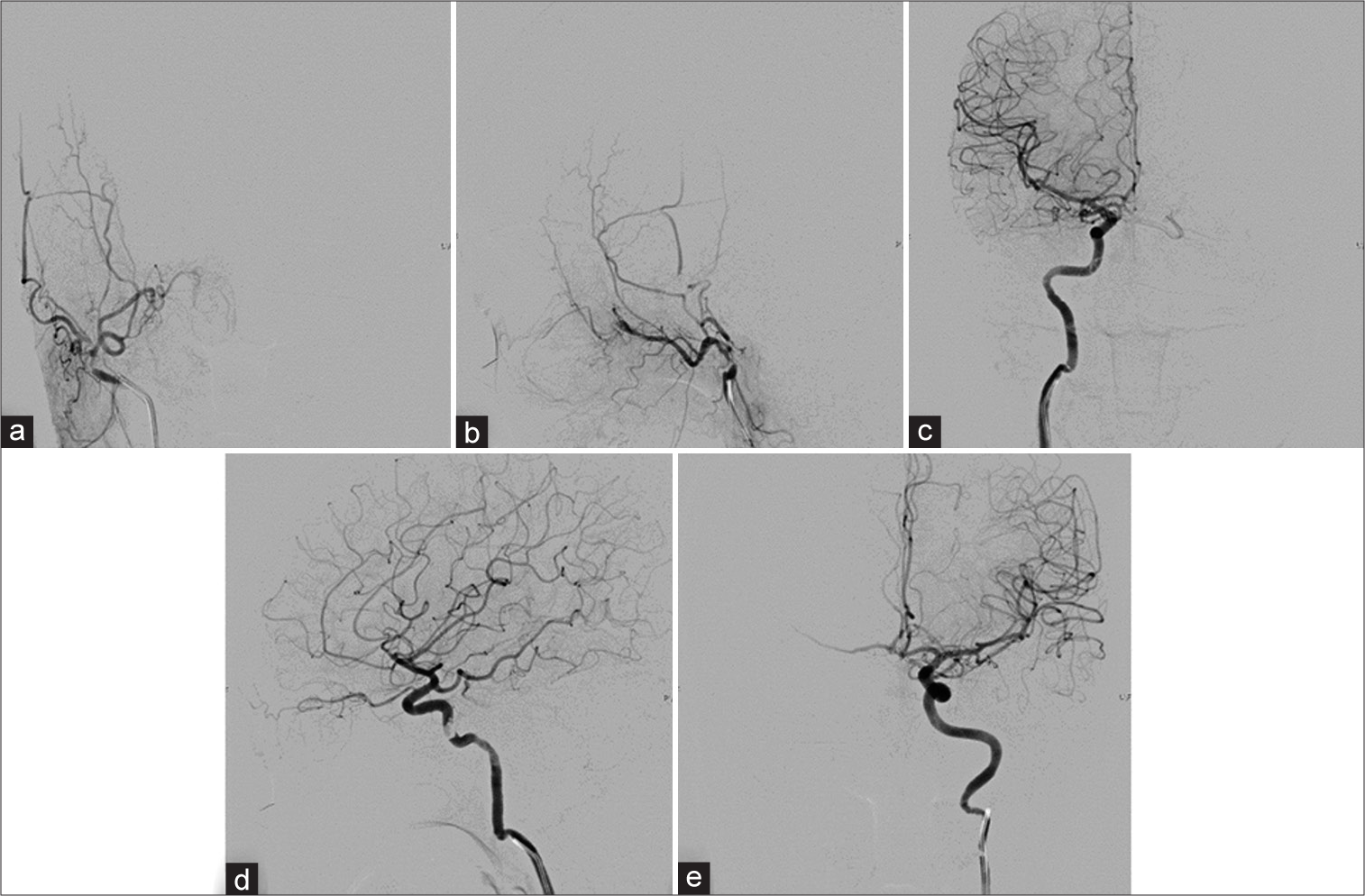
Figure 4:
(a) Anteroposterior (AP) and (b) lateral views of the right external carotid artery and (c) AP and (d) lateral views of the right internal carotid artery (ICA) injections show the right cavernous sinus dural arteriovenous fistula supplied from the right artery of the foramen rotundum, accessory meningeal artery, middle meningeal artery, and meningohypophyseal trunk (MHT) with drainage into the right dilated superficial middle cerebral and Labbé veins. There is no drainage into the right superior ophthalmic vein incompatible with the patient’s ocular symptoms, probably representing pre-existing thrombosis. (e) AP view of the left ICA injection demonstrates the clival branch from the left MHT supplying the fistula.
Figure 5:
(a) Anteroposterior (AP) and (b) lateral views of the right accessory meningeal artery injection show the right cavernous sinus dural arteriovenous fistula with drainage into the right dilated superficial middle cerebral and Labbé veins. (c) AP and (d) lateral views during Onyx injection. (e) AP and (f) lateral views of the right external carotid artery injection reveal incomplete obliteration of the fistula.
Figure 6:
Cerebral angiography obtained 6 months after embolization. (a) Anteroposterior (AP) and (b) lateral views of the right external carotid artery, (c) AP and (d) lateral views of the right internal carotid artery (ICA), and (e) AP view of the left ICA injections reveal complete regression of the right cavernous sinus dural arteriovenous fistula.
DISCUSSION
Due to the risk of embolic complications associated with transarterial embolization of CSDAVFs, a transfemoral transvenous approach through the ipsilateral IPS is recommended as the first-line access, whether the IPS is patent or occluded.[
Over the years, polyvinyl alcohol (PVA) particles and NBCA have frequently been used as embolic agents for CSDVAFs.[
CSDAVFs without cortical venous drainage have a relatively high incidence of spontaneous regression.[
To the best of our knowledge, spontaneous obliteration of malignant CSDAVFs after partial embolization through a transarterial route with liquid embolic materials has never been described in the available and relevant literature. In general, uncured CSDVAFs after transarterial embolization required further other therapeutic modalities, including transvenous and surgical approaches.[
The mechanism of further spontaneous regression of malignant CSDAVFs following partial transarterial embolization with liquid embolic material remains unclear.[
Spontaneous obliteration of low-flow CSDAVFs may result from partial or complete thrombosis of the CS and/or its tributaries.[
The findings of incomplete opacification of the CS and abnormal retrograde venous drainage are highly suspicious of partial thrombosis of the CS.[
Importantly, long-term follow-up of the incompletely cured patient is required for validation of the durability of the complete cure of the fistula.[
CONCLUSION
Spontaneous regression of malignant CSDAVFs following partial transarterial embolization is an exceedingly rare condition. In our two patients harboring low-flow CSDAVFs with preexisting thrombosis of the CS, it is possible that some parts of the liquid embolic materials could penetrate the fistulas, inducing thrombosis within the CS, resulting in complete closure of the fistulas.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alexander MD, Halbach VV, Hallam DK, Cooke DL, Ghodke BV, Dowd CF. Long-term outcomes of endovascular treatment of indirect carotid cavernous fistulae: Superior efficacy, safety, and durability of transvenous coiling over other techniques. Neurosurgery. 2019. 85: E94-100
2. Alexandre AM, Sturiale CL, Bartolo A, Romi A, Scerrati A, Flacco ME. Endovascular treatment of cavernous sinus dural arteriovenous fistulas. Institutional series, systematic review and meta-analysis. Clin Neuroradiol. 2022. 32: 761-71
3. Al-Afif S, Nakamura M, Götz F, Krauss JK. Spontaneous closure of a dural arteriovenous fistula. J NeuroInterv Surg. 2015. 7: e28
4. Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985. 62: 248-56
5. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995. 194: 671-80
6. Cognard C, Januel AC, Silva NA, Tall P. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: New management using Onyx. AJNR Am J Neuroradiol. 2008. 29: 235-41
7. Gross BA, Albuquerque FC, Moon K, McDougall CG. The road less traveled: Transarterial embolization of dural arteriovenous fistulas via the ascending pharyngeal artery. J Neurointerv Surg. 2017. 9: 97-101
8. Halbach VV, Higashida RT, Hieshima GB, Reicher M, Norman D, Newton TH. Dural fistulas involving the cavernous sinus: Results of treatment in 30 patients. Radiology. 1987. 163: 437-42
9. Iampreechakul P, Tirakotai W, Tanpun A, Wattanasen Y, Lertbusayanukul P, Siriwimonmas S. Spontaneous resolution of direct carotid-cavernous fistulas: Case series and literature review. Interv Neuroradiol. 2019. 25: 71-89
10. Iampreechakul P, Wangtanaphat K, Lertbutsayanukul P, Wattanasen Y, Siriwimonmas S. Spontaneous closure of a cavernous sinus dural arteriovenous fistula with spinal perimedullary drainage (Cognard V) during attempted transvenous embolization. Asian J Neurosurg. 2019. 14: 1268-74
11. Iampreechakul P, Wangtanaphat K, Hangsapruek S, Wattanasen Y, Lertbutsayanukul P, Siriwimonmas S. Transfemoral transvenous embolization through the vein of Trolard and superficial middle cerebral vein for cavernous sinus dural arteriovenous fistula with isolated cortical vein drainage: A case report and literature review. Surg Neurol Int. 2022. 13: 34
12. Iampreechakul P, Yuthagovit S, Wangtanaphat K, Hangsapruek S, Lertbutsayanukul P, Thammachantha S. Preoperative transarterial embolization of a large petrotentorial angiomatous meningioma using combination of liquid embolic materials: A case report. Asian J Neurosurg. 2022. 17: 500-6
13. Kai Y, Hamada J, Morioka M, Yano S, Kuratsu J. Treatment of cavernous sinus dural arteriovenous fistulae by external manual carotid compression. Neurosurgery. 2007. 60: 253-7
14. Liu HM, Huang YC, Wang YH, Tu YK. Transarterial embolisation of complex cavernous sinus dural arteriovenous fistulae with low-concentration cyanoacrylate. Neuroradiology. 2000. 42: 766-70
15. Liu HM, Wang YH, Chen YF, Cheng JS, Yip PK, Tu YK. Long-term clinical outcome of spontaneous carotid cavernous sinus fistulae supplied by dural branches of the internal carotid artery. Neuroradiology. 2001. 43: 1007-14
16. Liu P, Liu Y, Shi Y, An Q, Zhu W, Liu Y. The vascular architecture of cavernous sinus dural arteriovenous fistula and its impact on endovascular treatment approach selection and outcome. World Neurosurg. 2022. 166: e770-80
17. Luo CB, Chang FC, Teng MM, Lin CJ, Wang AG, Ting TW. Aggressive cavernous sinus dural arteriovenous fistula: Angioarchitecture analysis and embolization by various approaches. J Chin Med Assoc. 2016. 79: 152-8
18. Meyers PM, Halbach VV, Dowd CF, Lempert TE, Malek AM, Phatouros CC. Dural carotid cavernous fistula: Definitive endovascular management and long-term follow-up. Am J Ophthalmol. 2002. 134: 85-92
19. Rhim JK, Cho YD, Park JJ, Jeon JP, Kang HS, Kim JE. Endovascular treatment of cavernous sinus dural arteriovenous fistula with ipsilateral inferior petrosal sinus occlusion: A single-center experience. Neurosurgery. 2015. 77: 192-9
20. Ritchie WG, Lynch PR, Stewart GJ. The effect of contrast media on normal and inflamed canine veins. A scanning and transmission electron microscopic study. Invest Radiol. 1974. 9: 444-55
21. Satomi J, Satoh K, Matsubara S, Nakajima N, Nagahiro S. Angiographic changes in venous drainage of cavernous sinus dural arteriovenous fistulae after palliative transarterial embolization or observational management: A proposed stage classification. Neurosurgery. 2005. 56: 494-502
22. Seeger JF, Gabrielsen TO, Giannotta SL, Lotz PR. Carotid-cavernous sinus fistulas and venous thrombosis. AJNR Am J Neuroradiol. 1980. 1: 141-8
23. Viñuela F, Fox AJ, Debrun GM, Peerless SJ, Drake CG. Spontaneous carotid-cavernous fistulas: Clinical, radiological, and therapeutic considerations. Experience with 20 cases. J Neurosurg. 1984. 60: 976-84
24. Voigt K, Sauer M, Dichgans J. Spontaneous occlusion of a bilateral caroticocavernous fistula studied by serial angiography. Neuroradiology. 1971. 2: 207-11