- Department of Neurosurgery, Mohammed VI University Hospital center, Mohammed First University, Oujda, Morocco
Correspondence Address:
Mohamed Dahamou, Department of Neurosurgery, Mohammed VI University Hospital Center, Mohammed First University, Oujda, Morocco.
DOI:10.25259/SNI_303_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohamed Dahamou, Mohammed Alamine Elfarissi, Mohammed Lhamlili, Ibrahim Mehfoud, Mohamed Khoulali, Noureddine Oulali, Fayçal Moufid. Spontaneous regression of solid-cystic vestibular schwannoma: A case report. 09-Sep-2022;13:414
How to cite this URL: Mohamed Dahamou, Mohammed Alamine Elfarissi, Mohammed Lhamlili, Ibrahim Mehfoud, Mohamed Khoulali, Noureddine Oulali, Fayçal Moufid. Spontaneous regression of solid-cystic vestibular schwannoma: A case report. 09-Sep-2022;13:414. Available from: https://surgicalneurologyint.com/surgicalint-articles/11852/
Abstract
Background: Vestibular schwannomas (VSs) are one of the most common tumors of the cerebellopontine angle and internal meatus, the evolution of this type of tumors is defined as unpredictable, it can enlarge or present a spontaneous regression as described in rare cases.
Case Description: We report the case of a 50-year-old woman who presented with a large right full cystic VS revealed by a balance disorder associated with deafness in the right ear which spontaneously regressed. The patient was lost to follow-up for 3 years, the symptomatology improved, and the tumor clearly regressed without any surgical treatment.
Conclusion: Spontaneous regression of solid-cystic VS is possible but rare, it can be part of conservative treatment, which requires regular follow-up.
Keywords: Solid-cystic vestibular schwannoma, Spontaneous regression, Cerebellopontine angle tumors
INTRODUCTION
Vestibular schwannomas (VSs) are one of the most common tumors of the cerebellopontine angle (CPA) and internal meatus: they are benign and slow-growing tumors that arise from Schwann cells around the vestibular nerve and ganglia, which arise from the transition between central and peripheral myelin and may remain stable for many years.[
The evolution of this type of tumors is defined as unpredictable: it can enlarge or present a spontaneous regression as described in rare cases.[
They cause clinical symptoms by compressing and displacing adjacent structures in the internal auditory canal and CPA. The common symptom of revelation is hearing loss, but this can remain asymptomatic in many cases having been discovered by chance during radiological exploration, the confirmation of which is carried out by magnetic resonance imaging (MRI).[
The incidence of VS is increasing with an average of 1.3–2 new cases diagnosed per 100,000 people per year. The increasing detection rate is thought to be due to the widespread use of MRI.[
The choice of therapeutic modality is strongly influenced by the evolutionary patterns of VS and there is always a debate on the therapeutic choice after the diagnosis of VS, the choice of radiosurgery or surgery, or even management conservative may be an option depending on many factors, note that spontaneous regression may be observed in some cases but has already been described by some authors. The debate on the radiological aspects related to tumor involution has recently been revalued.[
We expect that some radiological features of the tumor may be features of spontaneous tumor regression.
We report a case of spontaneous regression of solid-cystic SV.
CASE REPORT
We report the case of a 50-year-old woman who consulted for a balance disorder associated with right deafness and a feeling of pressure around the left preauricular area.
She had no significant personal medical history or family medical history of neurocutaneous disorders.
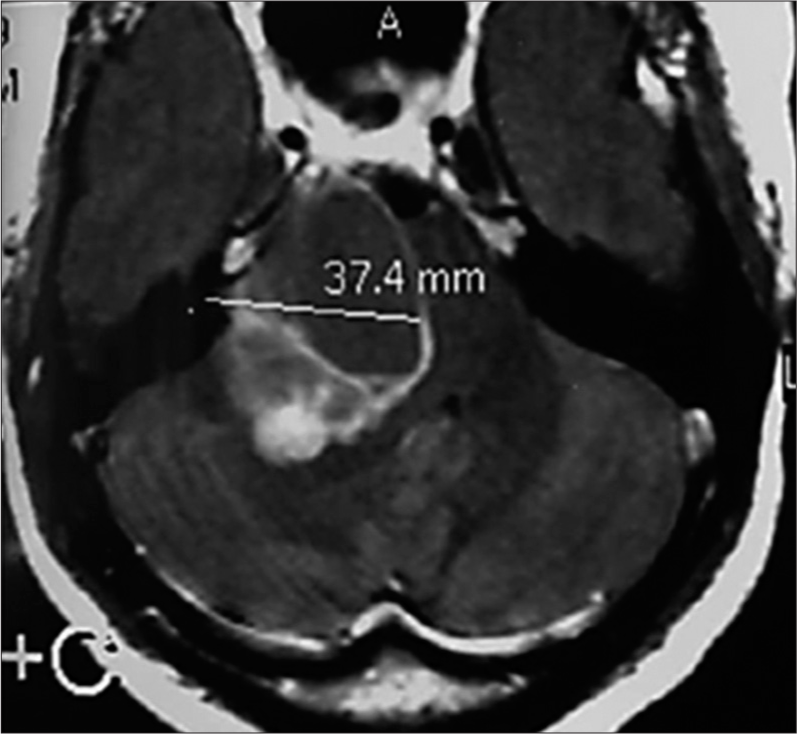
Her audiogram showed moderate sensorineural hearing loss on the right side and normal hearing on the left side. MRI was ordered which revealed a large right bicomponent CPA process: fleshy and cystic consistent with solid-cyclic VS [
The decision of surgical treatment was taken but the patient refused and was lost sight of.
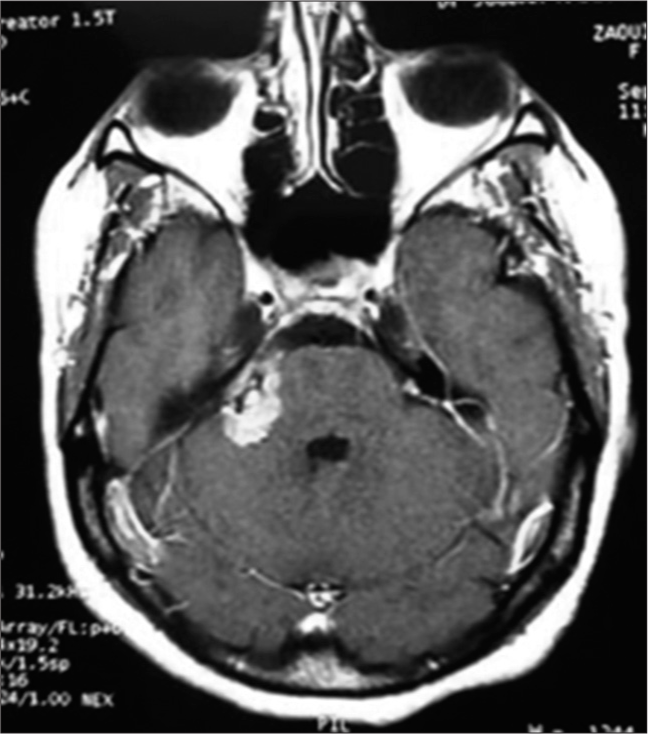
Three years later, the patient returned to our neurosurgery department, the examination of the patient showed a clear clinical improvement in the initial symptomatology, and a second MRI was performed which showed spontaneous regression of the cystic lesion of the tumor with persistence of a small fleshy residue [
DISCUSSION
VS is the most regular benign tumor of the CPA: it grows very slowly in general, and the growth rate can vary from 0.4 to 2.9 mm/year.[
The first symptom is hearing loss, and clinical examination may reveal other abnormalities such as nystagmus and balance disturbances, but some patients may remain asymptomatic, and radiological diagnosis can be obtained by MRI with contrast T1-weighted computed tomography (CT) scans for initial assessment. The MRI protocol should include taking T1- and T2-weighted sequences, diffusion-weighted imaging (DWI), and fluid-attenuated inversion recovery sequences. DWI imaging is useful in differentiating VS from arachnoid or epidermoid cysts. No less than a T2-weighted sequence is mandatory to exclude possible brainstem pathology mimicking signs of SV such as multiple sclerosis or glioma. The highly T2-weighted sub-millimeter axial sequence is the most important sequence for evaluating the vestibulocochlear nerve and its branches and illustrating the nerve as a hypointense linear structure bordering the hyperintense cerebrospinal fluid in adjacent cisterns (FIESTA, CISS, or DRIVE). CT participates in the evaluation of erythrocyte sedimentation rate. It provides practical preoperative knowledge on the surgical anatomy of the base of the skull, mainly the petrous bone.[
There are several ways to treat a VS, and we cite here: microsurgery, advances in stereotactic radiosurgery, and nonsurgical management with “watch, wait, and rescan,” especially in patients asymptomatic. It is generally agreed that conservative management is an appropriate option in patients with small SV (<15 mm). However, larger tumors are often considered for intervention at the time of initial presentation, although there is no consensus regarding an absolute tumor size when intervention should be considered, Stangerup et al. have suggested a surgery at the time of first presentation if the tumor is >15 mm in size. Ferri et al. have suggested intervention in patients with tumors larger than 20 mm in diameter, particularly in younger patients and those with poor hearing. However, Reddy et al. published a study on the results of conservative management of tumors with an intracranial diameter between 15 mm and 31 mm. They reported measurable growth in 24% of patients, no growth in 67%, and observed tumor regression in 9% of patients; they concluded that patients with medium or moderately large tumors can safely be offered an initial period of conservative management before intervention is considered.[
However, it appears that growth behavior can be unpredictable, with tumor growth occurring after a period of no growth and vice versa. The incidence of spontaneous regression varies in the literature, ranging from 1 to 30%. Relative regression rates also vary significantly from a marginal rate of 5% to complete tumor regression. In these reports, age, gender, tumor size, and location were unrelated to spontaneous shrinkage. In the largest study (n = 729) of the natural history of VS, Slangerup et al. reported a spontaneous size reduction of 0.9% of 322 extramental tumors. Other studies have reported shrinkage of VS in 1.2– 22% of VS.[
Almost all the studies reporting a sporadic decrease based their analyses on 2D postinjection T1-weighted imaging measurements. They reported between 3.8% and 13% spontaneous involution, with a basal shrinkage rate of between 0.74 and 1.6 mm/year. The discrepancies in these results can be explained by different sizing methods. Diagnosis of involution relies on 2D measurements, 3D measurements, and volumetric analysis which is the most accurate method to assess tumor size. Although not all cases are predictable, a first observation step can be accommodating when these radiological criteria are relevant. Eventually, if we assume nonsurgical treatment, close follow-up should be performed and any changes in clinical signs and symptoms should be promptly investigated and confirmed with magnetic resonance imaging. Aspects on tumor growth and radiological characteristics may be atypical but could help in decision-making.[
Spontaneous regression of solid-cystic VS is rare but possible. In our case, on a second MRI performed after 3 years, the disappearance of the cystic part of the tumor is observed.
The mechanism of tumor shrinkage is not well understood. Environmental factors and/or genetic-molecular assumptions must be considered. Until recently, no well-founded predictor of growth has been identified. Nevertheless, the neutrophilto-lymphocyte ratio has recently been presented as a valid predictive marker of VS growth, with a rate of 3.05 or higher having the best combination of sensitivity and specificity (83.8% and 100%, respectively).[
CONCLUSION
Spontaneous regression of solid-cystic VS is possible but rare, it can be part of conservative treatment, which requires regular follow-up.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amoo M, Rawluk D, Mcconn-Walsh R, Javadpour M. The shrinking vestibular schwannoma. Br J Neurosurg. 2019. p. 1-2
2. Battaglia A, Mastrodimos B, Cueva R. Comparison of growth patterns of acoustic neuromas with and without radiosurgery. Otol Neurotol. 2006. 27: 705-12
3. Bederson JB, von Ammon K, Wichmann WW, Yasargil MG. Conservative treatment of patients with acoustic tumors. Neurosurgery. 1991. 28: 646-50
4. Dunn IF, Bi WL, Mukundan S, Delman BN, Parish J, Atkins T. Congress of neurological surgeons systematic review and evidence-based guidelines on the role of imaging in the diagnosis and management of patients with vestibular schwannomas. Neurosurgery. 2018. 82: E32-4
5. El Bakkouri W, Kania RE, Guichard JP, Lot G, Herman P, Huy PT. Conservative management of 386 cases of unilateral vestibular schwannoma: Tumor growth and consequences for treatment. J Neurosurg. 2009. 110: 662-9
6. Ferri GG, Modugno GC, Pirodda A, Fioravanti A, Calbucci F, Ceroni AR. Conservative management of vestibular schwannomas: An effective strategy. Laryngoscope. 2008. 118: 951-7
7. Frisch CD, Jacob JT, Carlson ML, Foote RL, Driscoll CL, Neff BA. Stereotactic radiosurgery for cystic vestibular schwannomas. Neurosurgery. 2017. 80: 112-8
8. Hentschel M, Scholte M, Steens S, Kunst H, Rovers M. The diagnostic accuracy of non-imaging screening protocols for vestibular schwannoma in patients with asymmetrical hearing loss and/or unilateral audiovestibular dysfunction: A diagnostic review and meta-analysis. Clin Otolaryngol. 2017. 42: 815-23
9. Huang X, Caye-Thomasen P, Stangerup SE. Distinct spontaneous shrinkage of a sporadic vestibular schwannoma. Auris Nasus Larynx. 2013a. 40: 243-6
10. Huang X, Caye-Thomasen P, Stangerup SE. Spontaneous tumour shrinkage in 1261 observed patients with sporadic vestibular schwannoma. J Laryngol Otol. 2013b. 127: 739-43
11. Kontorinis G, Crowther JA, Iliodromiti S, Taylor WA, Locke R. Neutrophil to lymphocyte ratio as a predictive marker of vestibular schwannoma growth. Otol Neurotol. 2016. 37: 580-5
12. Lahlou G, Rodallec M, Nguyen Y, Sterkers O, Kalamarides M. How to radiologically identify a spontaneous regression of sporadic vestibular schwannoma?. PLoS One. 2019. 14: e0217752
13. Luetje CM. Spontaneous involution of acoustic tumors. Am J Otol. 2000. 21: 393-8
14. Marinelli JP, Lees KA, Lohse CM, Driscoll CL, Neff BA, Link MJ. Natural history of growing sporadic vestibular schwannomas: An argument for continued observation despite documented growth in select cases. Otol Neurotol. 2020. 41: e1149-53
15. Nikolopoulos TP, Fortnum H, O’Donoghue G, Baguley D. Acoustic neuroma growth: A systematic review of the evidence. Otol Neurotol. 2010. 31: 478-85
16. Pennings RJ, Morris DP, Clarke L, Allen S, Walling S, Bance ML. Natural history of hearing deterioration in intracanalicular vestibular schwannoma. Neurosurgery. 2011. 68: 68-77
17. Reddy CE, Lewis-Jones HG, Javadpour M, Ryland I, Lesser TH. Conservative management of vestibular schwannomas of 15 to 31 mm intracranial diameter. J Laryngol Otol. 2014. 128: 752-8
18. Romani R, Pollock J. Spontaneous shrinkage of vestibular schwannoma. Surg Neurol Int. 2016. 7: 59
19. Smouha EE, Yoo M, Mohr K, Davis RP. Conservative management of acoustic neuroma: A meta-analysis and proposed treatment algorithm. Laryngoscope. 2005. 115: 450-4
20. Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J. The natural history of vestibular schwannoma. Otol Neurotol. 2006. 27: 547-52
21. Stangerup SE, Tos M, Caye-Thomasen P, Tos T, Klokker M, Thomsen J. Increasing annual incidence of vestibular schwannoma and age at diagnosis. J Laryngol Otol. 2004. 118: 622-7
22. Tos M, Stangerup SE, Cayé-Thomasen P, Tos T, Thomsen J. What is the real incidence of vestibular schwannoma?. Arch Otolaryngol Head Neck Surg. 2004. 130: 216-20
23. Tschudi DC, Linder TE, Fisch U. Conservative management of unilateral acoustic neuromas. Am J Otol. 2000. 21: 722-8
24. Yoshimoto Y. Systematic review of the natural history of vestibular schwannoma. J Neurosurg. 2005. 103: 59-63