- Department of Neurosurgery, Kitano Hospital, Tazuke Kofukai Medical Research Institute, Osaka, Japan.
- Department of Neurosurgery, Hikari Hospital, Otsu City, Shiga, Japan.
- Department of Neurosurgery, Kurashiki Central Hospital, Kurashiki, Okayama, Japan.
Correspondence Address:
Ryota Ishibashi, Department of Neurosurgery, Kitano Hospital, Tazuke Kofukai Medical Research Institute, Osaka, Japan.
DOI:10.25259/SNI_610_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ryota Ishibashi1, Yoshinori Maki2, Hiroyuki Ikeda3, Masaki Chin3. Spontaneous resolution of a tentorial dural arteriovenous fistula fed by the artery of Wollschlaeger and Wollschlaeger after embolization of the main shunting point. 16-Aug-2021;12:413
How to cite this URL: Ryota Ishibashi1, Yoshinori Maki2, Hiroyuki Ikeda3, Masaki Chin3. Spontaneous resolution of a tentorial dural arteriovenous fistula fed by the artery of Wollschlaeger and Wollschlaeger after embolization of the main shunting point. 16-Aug-2021;12:413. Available from: https://surgicalneurologyint.com/surgicalint-articles/11042/
Abstract
Background: Tentorial dural arteriovenous fistula (TDAVF) is a rare intracranial vascular shunt. A TDAVF can be supplied by the Artery of Wollschlaeger and Wollschlaeger (AWW). However, a limited number of cases of TDAVF fed by the AWW have been reported to date.
Case Description: A 70-year-old woman complaining of the right motor weakness underwent magnetic resonance imaging. A vascular lesion beneath the cerebellar tentorium was incidentally found with chronic infarction of the left corona radiata. Angiographically, the vascular lesion was a TDAVF supplied by the bilateral posterior meningeal arteries. No other apparent feeders were detected. The TDAVF had a shunting point on the inferior surface of the cerebellar tentorium with venous retrograde flow (Borden type III, Cognard type III). To prevent vascular events, endovascular embolization was performed using n-butyl-2-cyanoacrylate. Following embolization of the shunting point, a residual shunt fed by the AWW was identified. The shunt supplied by the AWW was not observed preoperatively. Follow-up angiography performed 1 week later revealed spontaneous disappearance of the residual shunt. The patient was followed-up in our outpatient clinic, and no recurrence of the TDAVF was confirmed postoperatively.
Conclusion: Detection of mild feeding from the AWW to a TDAVF can be elusive preoperatively. Following embolization of the main shunting point, residual shunting from the AWW can resolve spontaneously.
Keywords: Artery of Wollschlaeger and Wollschlaeger, Endovascular treatment, N-butyl-2-cyanoacrylate, Tentorial dural arteriovenous fistula
INTRODUCTION
Intracranial dural arteriovenous fistulas account for approximately 10–15% of intracranial arteriovenous malformations.[
Herein, we present a rare case of TDAVF fed by the AWW recognized after endovascular embolization of the main shunting point fed by the bilateral posterior meningeal arteries (PMAs).
CASE PRESENTATION
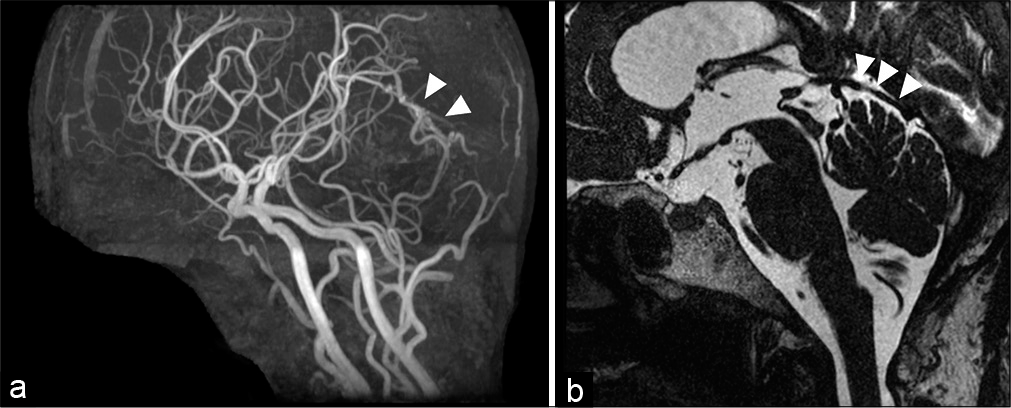
A 70-year-old woman presented to our outpatient clinic. She complained of motor weakness in the right hemisphere, which had appeared 4 months before. Magnetic resonance imaging was performed to rule out the presence of any intracranial lesions. Chronic lacunae infraction of the left corona radiata was detected on fluid-attenuated inversion recovery images. The lacunae infraction of the left corona radiata was considered a cause of her right motor weakness. No edematous changes were found in the infratentorial or supratentorial regions. However, magnetic resonance angiography revealed a vascular lesion [
Figure 2:
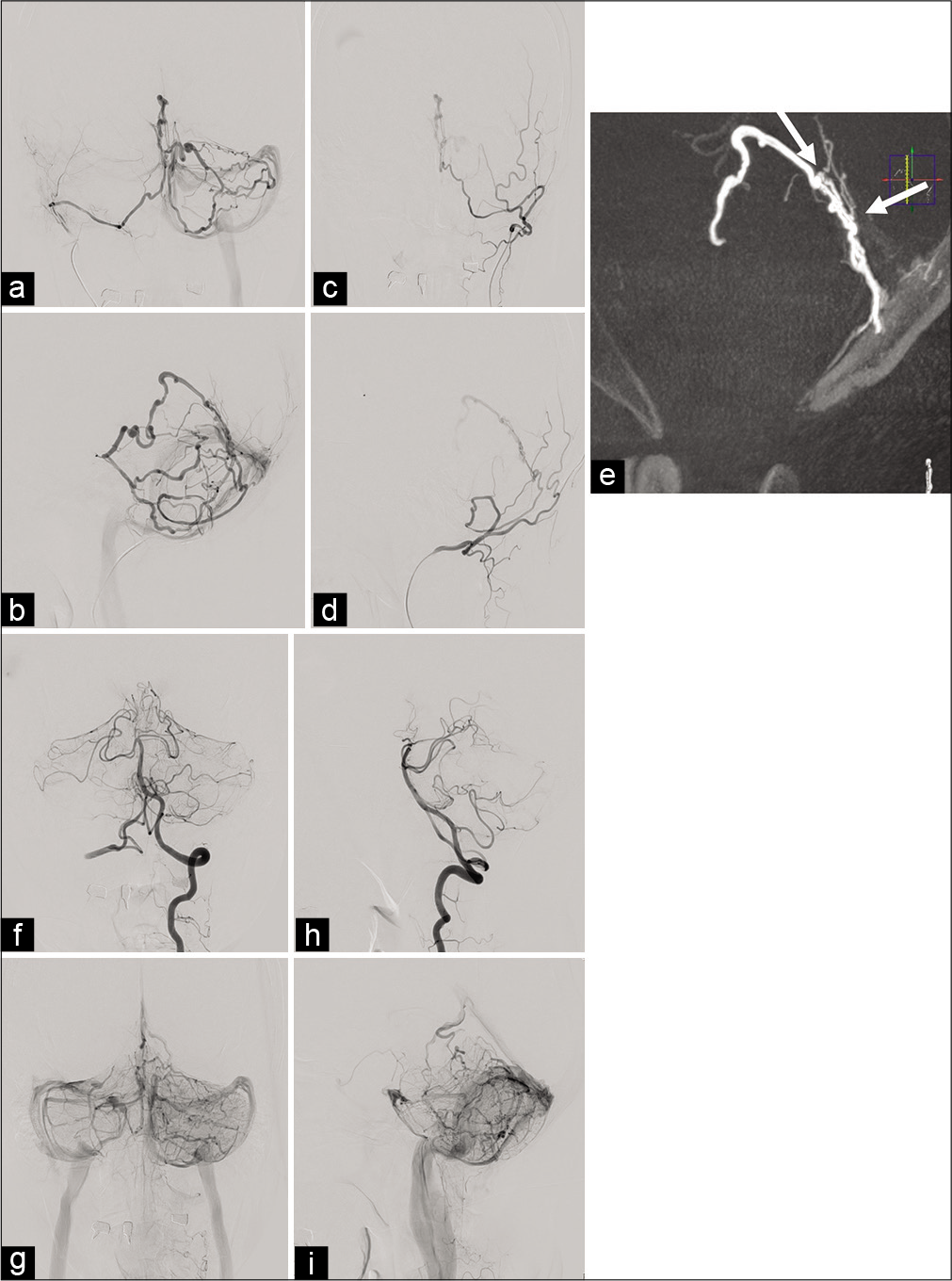
Preoperative angiographic findings. Selective angiogram of the right mastoid branch of the occipital artery demonstrating an arteriovenous shunt fed by the right PMA. The shunt shows two drainage pathways, running through the superior vermian vein to the petrosal vein terminating to the transvers sinus and through the superior vermian vein to the inferior vermian vein terminating to the tentorial sinus (a: anteroposterior projection and b: lateral projection). A left occipital angiogram reveals that the arteriovenous shunt is also supplied by the left PMA (c: anteroposterior projection and d: lateral projection). The shunting point is located beneath the cerebellar tentorium (white arrows: caliber change) (e). A left vertebral angiogram does not reveal any apparent feeding arteries. Venous congestion of the left vermian hemisphere is observed. The venous circulation is antegrade but stagnated. (f) Arterial phase of anteroposterior projection. (g) Venous phase of anteroposterior projection (h) arterial phase of lateral projection. (i) Venous phase of lateral projection. PMA: Posterior meningeal artery.
Endovascular surgery
Under general anesthesia, two Envoy 5F 90 cm MPD (Codman Neuro, Raynham, MA, USA) were introduced in the bilateral occipital artery. The mastoid branch of the left occipital artery was selected with a Masters HF 2.8-Fr/3.2-Fr 125 cm (ASAHI INTECC J-Sales, CO., LTD, Tokyo, Japan) and a Traxcess 14 0.012/0.014 200 cm (Terumo Corporation, Tokyo, Japan). The Masters HF 2.8-Fr/3.2-Fr 125 cm was placed at the mastoid foramen. Using an ASAHI CHIKAI 0.014 200 cm (ASAHI INTECC J-Sales, CO., LTD, Tokyo, Japan) and a Traxcess 14 0.012/0.014 200 cm, another Masters HF 2.8-Fr/3.2-Fr 125 cm was introduced through the mastoid branch of the right occipital artery to the right PMA. As the left PMA was quite tortuous compared to the right PMA, we considered that a transarterial embolization approached from the right PMA was the easiest course of action. From the right Masters HF 2.8-Fr/3.2-Fr 125 cm, a Carnelian MARVEL® 1.6/1.8-Fr 155 cm (Tokai Medical Products Inc.) was introduced near the shunting point with an ASAHI CHIKAI 0.008 200 cm (ASAHI INTECC J-Sales, CO., LTD, Tokyo, Japan) [
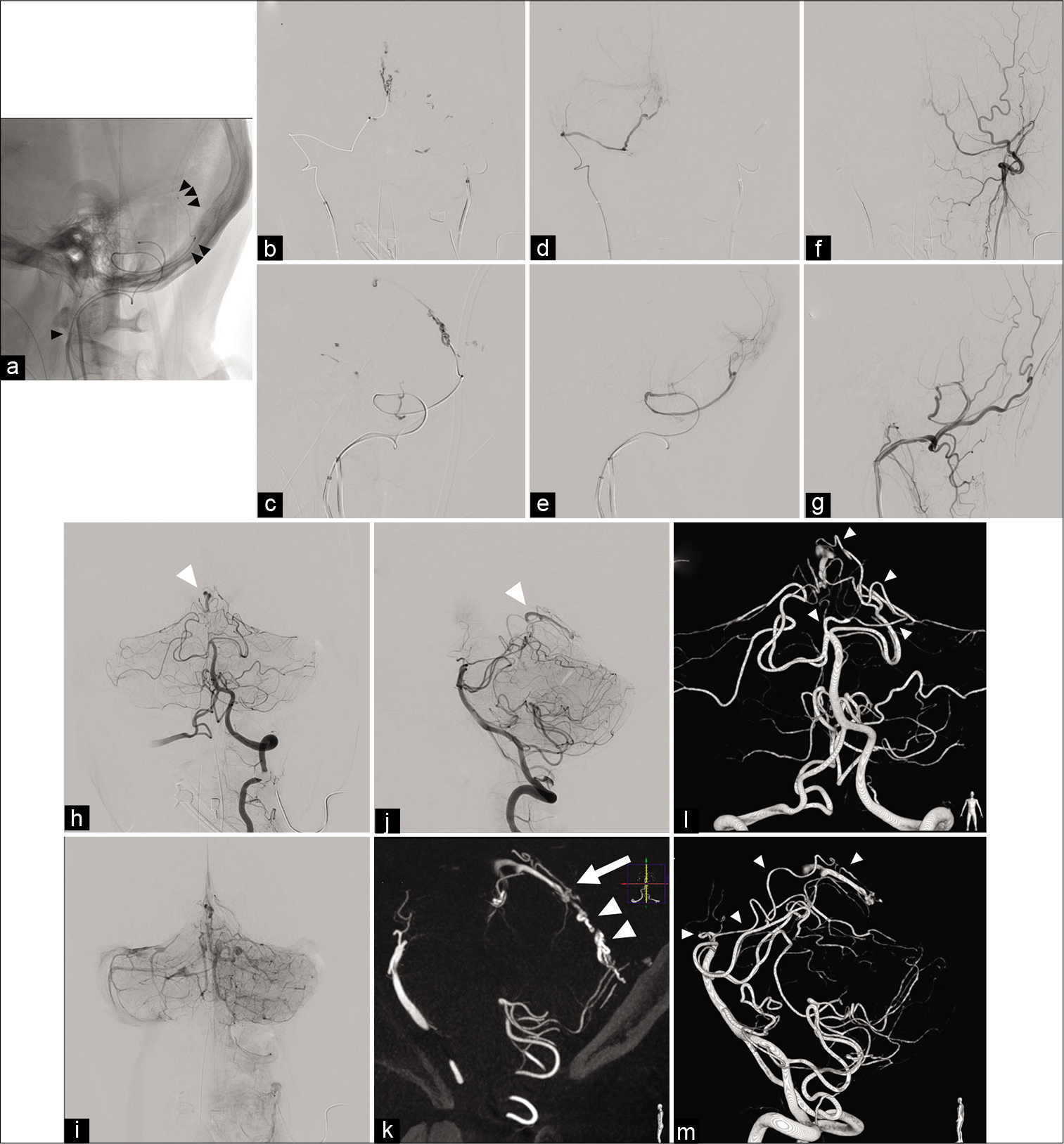
Figure 3:
Intraoperative angiographic findings. Endovascular devices were introduced near to the shunting point (single black arrowhead: Envoy 5F 90 cm MPD, double black arrow heads: Masters HF 2.8-Fr/3.2-Fr 125 cm and triple black arrow heads: Carnelian MARVEL® 1.6/1.8-Fr 155 cm) (a: lateral projection). The shunting point was embolized with 20% of NBCA. NBCA was partially migrated (b: anteroposterior injection and c: lateral projection). After the injection of 20% NBCA, the arteriovenous shunt was not seen. (d) Anteroposterior projection of the right occipital angiogram. (e) Lateral projection of the right occipital angiogram. (f) Anteroposterior projection of the left occipital angiogram. (g) Lateral projection of the left occipital angiogram. The left vertebral angiogram shows the residual arteriovenous shunt (white arrowhead). Venous congestion remained (h: arterial phase of anteroposterior projection, i: venous phase of anteroposterior projection, and j: arterial phase of lateral projection). The residual shunting point is located beneath the cerebellar tentorium (white arrow). The shunting point embolized with 20% of NBCA is also recognized (white arrow heads) (k: a sagittal image of maximum intensity projection). The residual arteriovenous shunt is supplied by the AWW (white arrow heads). The AWW branched near the bifurcation of the basilar artery and superior cerebellar artery (l and m: three-dimensional images). AWW: The artery of Wollschlaeger and Wollschlaeger, NBCA: n-butyl-2-cyanoacrylate.
On the 3D-MIP images, a dural branch originating from the SCA was identified as the feeder of the residual shunt. The artery originated close to the bifurcation of the basilar artery and the SCA. The artery branched from the anterior pontomesencephalic segment of the SCA and was considered to be the AWW [
Postoperative course
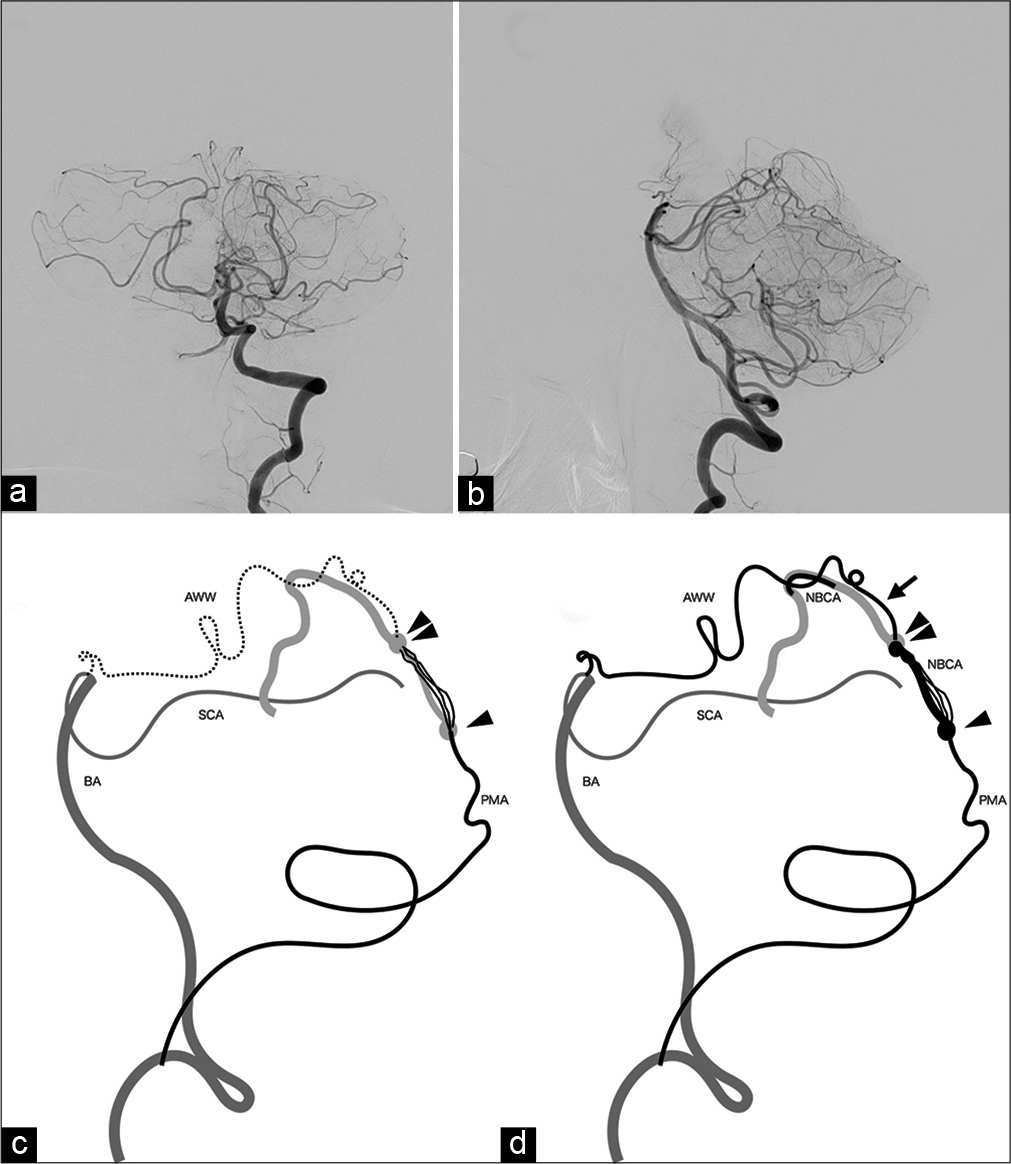
The patient’s postoperative course was uneventful. The residual arteriovenous fistula fed by the AWW disappeared spontaneously, as seen on follow-up vertebral angiography 1 week after the procedure [
Figure 4:
Postoperative angiography and schemas of the TDAVF in this case. (a and b) Left vertebral angiogram performed 1 week after the endovascular surgery reveals no residual arteriovenous shunt. (c and d) Schemas of the arterial supply and shunting point of the TDAVF. Preoperatively, the arterial feeding by AWW was not visualized (c). Postoperatively, the arterial feeding by AWW is observed (arrow). The shunting point was embolized with NBCA (the region margined with arrow heads). AWW: The artery of Wollschlaeger and Wollschlaeger, BA: The basilar artery, NBCA: n-butyl-2-cyanoacrylate, PMA: The posterior meningeal artery, SCA: The superior cerebellar artery.
DISCUSSION
Here, we present a relatively rare case of TDAVF fed by bilateral PMAs and AWW. Our case showed that the arterial supply from AWW to TDAVF was not visualized preoperatively. However, the arterial supply from the AWW to TDAVF was clearly observed after the bilateral PMAs were embolized with NBCA. In addition, the residual shunt fed by the AWW disappeared spontaneously without a second surgical treatment.
The following arteries supplying the cerebellar tentorium can feed a TDAVF: the meningeal branches of the internal carotid artery (meningohypophyseal trunk), external carotid artery (ascending pharyngeal artery, occipital artery, and middle meningeal artery), posterior cerebellar artery (artery of Davidoff and Schechter), and vertebral artery (PMA).[
The arterial supply to the cerebellar tentorium by SCA is inconsistent and frequently overlooked.[
In the present case, the residual shunt fed by the AWW resolved spontaneously after the shunting point was embolized with NBCA. This is possibly because the shunting point between the bilateral PMAs and the tentorial sinus was the main pathological factor. Subsequently, the residual shunt could have been thrombosed. This speculation seems coherent with preoperative angiographic findings in that the arterial supply by the bilateral PMAs was visualized while no arterial supply by the AWW was observed [
CONCLUSION
Here, we present a rare case of TDAVF supplied by bilateral PMAs and AWW. Mild supply to the TDAVF can be visualized after the major shunting point is embolized. Surgeons should always be aware of this possibility and should assess the necessity of additional embolization of the residual arterial supply by AWW. Postoperative angiographical follow-up can be sufficient in cases in which the flow of the residual shunt is low.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Awad IA, Little JR, Akarawi WP, Ahl J. Intracranial dural arteriovenous malformations: Factors predisposing to an aggressive neurological course. J Neurosurg. 1990. 72: 839-50
2. Benner D, Hendricks BK, Benet A, Lawton MT. Eponyms in vascular neurosurgery: Comprehensive review of 11 arteries. World Neurosurg. 2021. 151: 249-57
3. Byrne JV, Garcia M. Tentorial dural fistulas: Endovascular management and description of the medial dural-tentorial branch of the superior cerebellar artery. Am J Neuroradiol. 2013. 34: 1798-804
4. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995. 194: 671-80
5. Gioppo A, Faragò G, Caldiera V, Caputi L, Cusin A, Ciceri E. Medial tentorial dural arteriovenous fistula embolization: Single experience with embolic liquid polymer SQUID and review of the literature. World Neurosurg. 2017. 107: 1050.e1-7
6. Krafft PR, Liu SS, Agarwalla PK, Van Loveren HR. The meningeal branch of the superior cerebellar artery. Br J Neurosurg. 2018. 32: 273-5
7. Kvint S, Ramchand P, Cox M, Sedora-Roman NI, Bagley L, O’Rourke DM. Type V dural arteriovenous fistula supplied by the artery of Wollschlaeger and Wollschlaeger causing cervical myelopathy and quadriparesis. World Neurosurg. 2020. 137: 55-61
8. Lawton MT, Sanchez-Mejia RO, Pham D, Tan J, Halbach VV. Tentorial dural arteriovenous fistulae: Operative strategies and microsurgical results for six types. Neurosurgery. 2008. 62: 110-24
9. Meybodi AT, Vigo V, Lawton MT, Benet A. The artery of Wollschlaeger and Wollschlaeger: An anatomical-clinical illustration. Br J Neurosurg. 2017. 31: 593-5
10. Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology. 1969. 93: 1071-8
11. Picard L, Bracard S, Islak C, Roy D, Moreno A, Marchal JC. Dural fistulae of the tentorium cerebelli. Radioanatomical clinical and therapeutic considerations. J Neuroradiol. 1990. 17: 161-81
12. Puffer RC, Daniels DJ, Kallmes DF, Cloft HJ, Lanzino G. Curative Onyx embolization of tentorial dural arteriovenous fistulas. Neurosurg Focus. 2012. 32: E4
13. Wollschlaeger PB, Wollschlaeger G. An infratentorial meningeal artery. Radiologe. 1965. 5: 451-2
14. Zhou LF, Chen L, Song DL, Gu YX, Leng B. Tentorial dural arteriovenous fistulas. Surg Neurol. 2007. 67: 472-81