- Department of Neurosurgery, National Hospital Organization Osaka National Hospital, Osaka, Japan.
Correspondence Address:
Tomohiko Ozaki, Department of Neurosurgery, National Hospital Organization Osaka National Hospital, Osaka, Japan.
DOI:10.25259/SNI_529_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hiroki Yamazaki, Toshiyuki Fujinaka, Tomohiko Ozaki, Tomoki Kidani, Keisuke Nishimoto, Kowashi Taki, Naoki Nishizawa, Keijiro Murakami, Yonehiro Kanemura, Shin Nakajima. Staged treatment for ruptured wide-neck intracranial aneurysm with intentional partial coiling in the acute phase followed by definitive treatment. 22-Jul-2022;13:322
How to cite this URL: Hiroki Yamazaki, Toshiyuki Fujinaka, Tomohiko Ozaki, Tomoki Kidani, Keisuke Nishimoto, Kowashi Taki, Naoki Nishizawa, Keijiro Murakami, Yonehiro Kanemura, Shin Nakajima. Staged treatment for ruptured wide-neck intracranial aneurysm with intentional partial coiling in the acute phase followed by definitive treatment. 22-Jul-2022;13:322. Available from: https://surgicalneurologyint.com/surgicalint-articles/11728/
Abstract
Background: Evidence supports endovascular coiling for ruptured intracranial aneurysms (RIAs). However, in some cases, it is difficult to achieve complete occlusion by coiling, such as with wide-neck aneurysms. We report our experience with intentional staged RIA treatment using targeted endovascular coiling at the rupture point in the acute phase, followed by delayed stent-assisted coiling, flow diverter stenting, or surgical clipping.
Methods: Consecutive patients with RIAs treated between April 2015 and June 2021 were retrospectively investigated. Clinical characteristics, treatment complications, and patient outcomes data were collected.
Results: Among 108 RIAs treated in our hospital, 60 patients underwent initial coiling; 10 patients underwent staged treatment. The aneurysm locations were the anterior communicating artery (n = 5), internal carotid-posterior communicating artery (n = 3), internal carotid-paraclinoid (n = 1), and vertebral artery-posterior inferior cerebellar artery (n = 1). The mean ± standard deviation aneurysmal diameter was 9.6 ± 5.4 mm and the mean aspect ratio was 1.2 ± 0.7. As the second treatment to obliterate blood flow to the neck area, we performed five stent-assisted coiling, two flow-diverter stentings, and three surgical clippings. Only one minor perioperative complication occurred. The median duration between the first and second treatments was 18 days (range, 14– 42 days). Good clinical outcome (modified Rankin scale score 0–2) at 90 days was achieved in 5 (50%) cases. The median follow-up duration was 6.5 months (range, 3–35 months); no rerupture occurred.
Conclusion: Intentional staged treatment with a short time interval for RIA was effective and feasible.
Keywords: Coil embolization in acute phase, Ruptured intracranial aneurysm, Staged treatment
INTRODUCTION
Surgical clipping for ruptured intracranial aneurysms (RIA) is a well-established treatment.[
Evidence supporting endovascular coiling for RIA has been established.[
Although stent-assisted coil embolization is effective in wide-neck aneurysms, there is no good evidence supporting stent-assisted coiling in RIA due to the high complication rate, especially thromboembolic complications under an insufficient effect of antiplatelet agents.[
MATERIALS AND METHODS
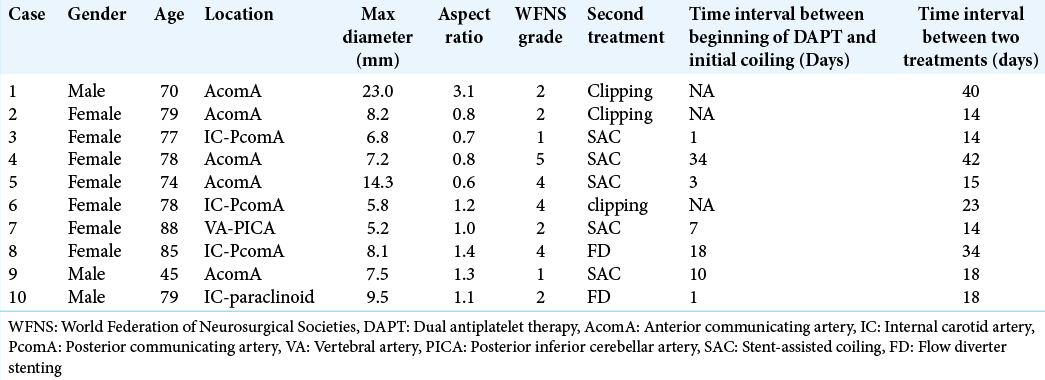
This study was approved by the Institutional Ethics Committee of our institution and was performed in accordance with the committee’s guidelines (Approval number: 18025). All patients provided consent. Using a prospectively collected neurovascular database, consecutive patients with RIAs managed at our hospital between April 2015 and June 2021 were retrospectively investigated. All patients who underwent intentional staged treatment for RIAs with targeted endovascular embolization at the ruptured point in the acute phase followed by a second treatment with stent-assisted embolization, FD stenting, or surgical clipping were enrolled in this study. In all cases, operations were performed under general anesthesia and patients were kept under severe sedation for a few days after initial embolization to prevent rerupture, as we considered that thromboses of the aneurysms were occurred during those days. The second treatment was selected based on imaging evaluation at the time of presentation. The patients’ clinical characteristics were collected, namely, age, sex, location of the aneurysm, World Federation of Neurosurgical Societies (WFNS) grade, treatment complications, days between the two treatments, and final modified Rankin scale (mRS) score.
RESULTS
Between April 2015 and June 2021, 108 RIAs were treated in our hospital. Sixty patients were treated with coil embolization, and 10 of these patients underwent staged treatment [
Treatment, complications, and clinical outcomes
As the second treatment to obliterate blood flow to the neck area, five stent-assisted coil embolization, three surgical clippings, and two FD stentings were performed. In the 20 treatment procedures (10 cases undergoing two procedures), only one perioperative complication occurred. Specifically, intraparenchymal hemorrhage occurred about 10 h after stent-assisted coil embolization for an AcomA aneurysm; however, the patient was discharged with an mRS score of 1. The median duration between the first and second treatments was 18 days (range, 14–42 days). Rerupture between these treatments did not occur. Good clinical outcome, defined as an mRs score of 0–2, at 90 days, was achieved in 5 (50%) cases. The median follow-up duration was 6.5 months (range, 3–35 days), with no rerupture. Recanalization occurred in one case 8 months after stent-assisted coil embolization for an AcomA aneurysm. Additional embolization was performed successfully, and the patient has been free from complications.
Representative cases
Case 4
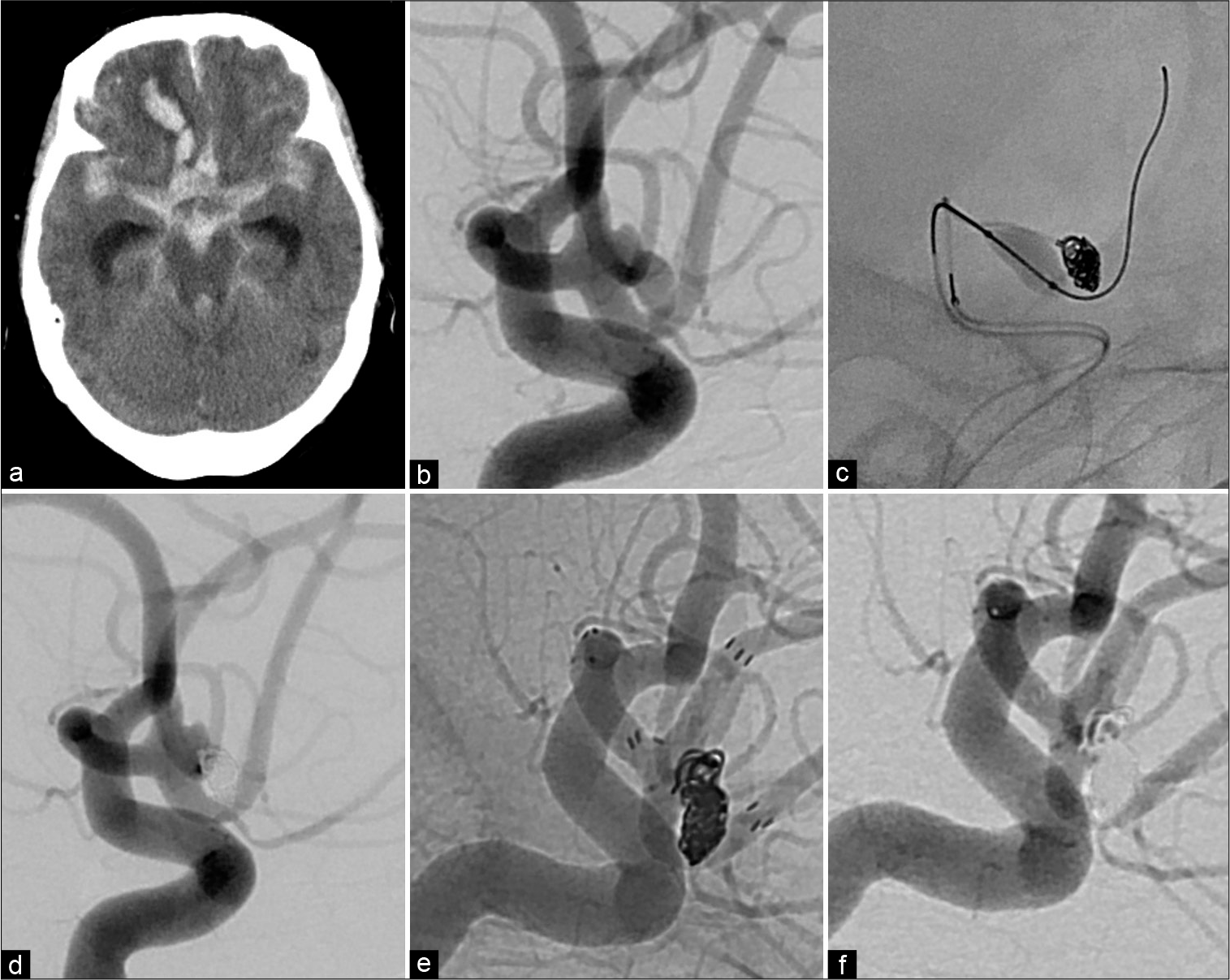
A 78-year-old female patient was brought to our hospital because of impaired consciousness. Computed tomography (CT) and CT angiography showed subarachnoid hemorrhage (SAH) due to rupture of a 4 mm AcomA aneurysm; the WFNS grade was 5 [
Figure 2:
Representative case. (a) Computed tomography image showing diffuse subarachnoid hemorrhage and intraparenchymal hemorrhage in the right frontal lobe. (b) Working projection of the initial angiogram. (c) Working projection of the angiogram obtained during the first treatment showing coil embolization with balloon-assisted coil embolization. (d) Three-dimensional reconstruction image of the final angiogram of the first treatment showing obstruction of the aneurysmal dome except for the neck region. (e) Working projection of the final angiogram (native image) of the definitive treatment showing obstruction of the entire aneurysmal dome. (f) Working projection of the final angiogram of the definitive treatment showing obstruction of the entire aneurysmal dome.
Case 6
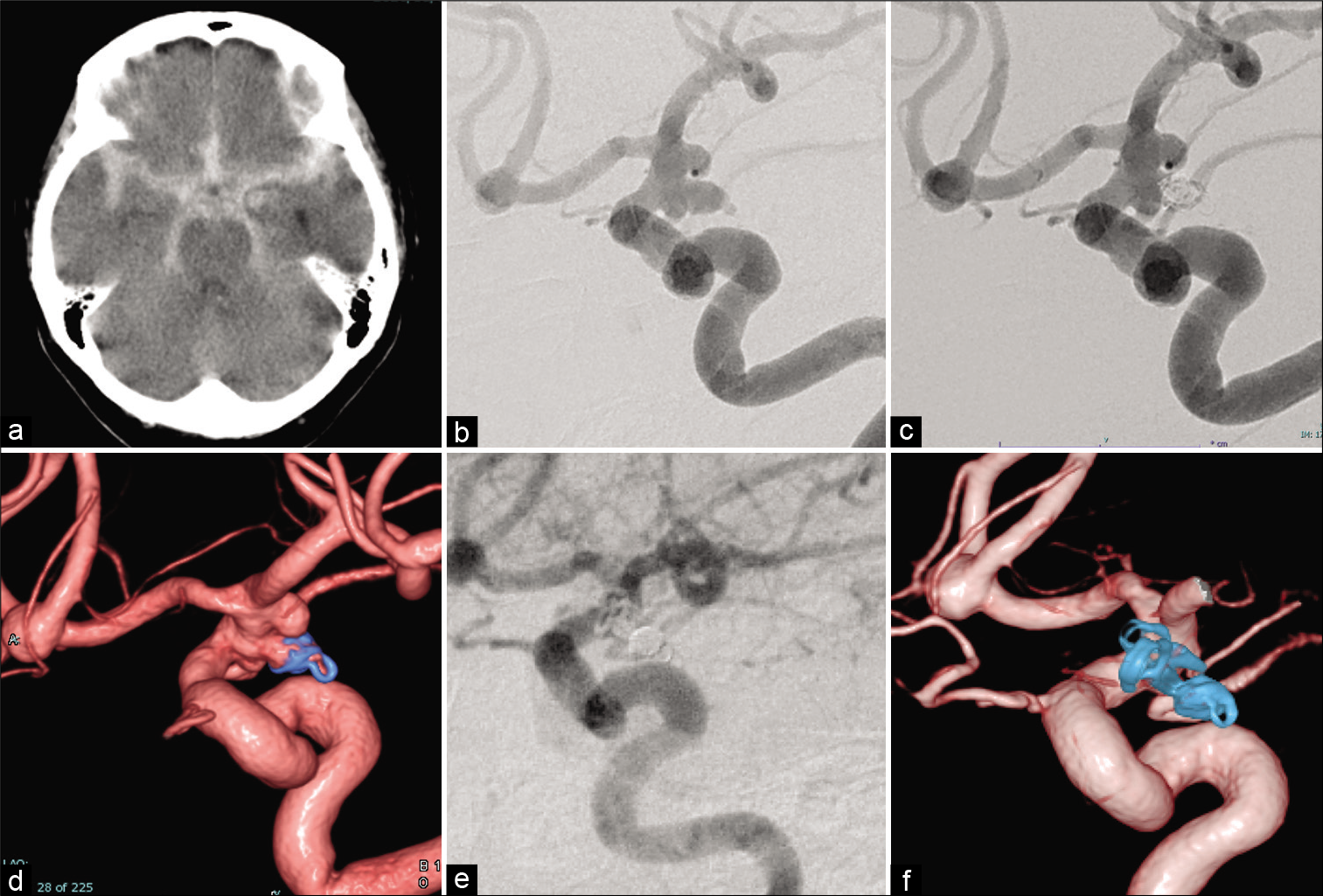
A 78-year-old female was brought to our hospital because of impaired consciousness. CT and CT angiography showed SAH due to rupture of a 6.5 mm left IC-PcomA aneurysm; the WFNS grade was 4 [
Figure 3:
Representative case. (a) Computed tomography showing diffuse subarachnoid hemorrhage. (b) Working projection of the initial angiogram. The arrow shows the aneurysmal bleb (presumed rupture point). (c) Working projection of the final angiogram of the first treatment showing obstruction of the aneurysmal dome except for the neck region. (d) Three-dimensional reconstruction image of the final angiogram of the first treatment showing obstruction of the aneurysmal dome except for the neck region. (e) Working projection of the final angiogram of the definitive treatment showing obstruction of the entire aneurysmal dome. (f) Three-dimensional reconstruction image of the final angiogram of the definitive treatment showing obstruction of the entire aneurysmal dome.
DISCUSSION
The present study demonstrated the feasibility and efficacy of intentional staged treatment for RIA with targeted endovascular embolization at the rupture point in the acute phase followed by a second treatment comprising stent-assisted embolization, FD stenting, or surgical clipping.
Surgical clipping is a well-established treatment for RIAs;[
The International Subarachnoid Aneurysm Trial concluded that the survival rate and favorable outcome (mRS score: 0–2) rate 10 years after SAH treated with coil embolization are higher than those obtained after surgical clipping.[
Considering previous findings, staged treatment of ruptured wide-neck aneurysm by placing coils as safely as possible without stents in the acute phase and treating the remaining area around the aneurysmal neck by clipping, stent-assisted coiling, or FD stenting might be an effective treatment concept.[
Using vessel wall magnetic resonance imaging, wall enhancement of a cerebral aneurysm has been revealed as a characteristic of ruptured aneurysms.[
Limitations
The most critical limitation of this study is its retrospective nature, which may have resulted in selection bias. However, there were no failed cases among the intentional staged treatment cases. In addition, this study reviewed patients over an approximately 5-year period, during which new therapeutic devices and techniques were developed, particularly FD stents. Considering this limitation, treatment bias could not be avoided. Finally, this case series is limited by its small sample size. There were only a few aneurysms in each location, and the appropriate second treatment method and our conclusions require a large prospective study for confirmation.
CONCLUSION
Intentional staged treatment of RIAs with coil embolization in the acute phase followed by a second treatment with stent-assisted embolization, FD stenting, or surgical clipping was effective and feasible with a short time interval. This strategy could be an option for cases in which both surgical clipping and primary coil embolization are challenging.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Grant-in-Aid for Early-Career Scientists from the Japan Society for the Promotion of Science (18K16582).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This work was supported by a Grant-in-Aid for Early-Career Scientists from the Japan Society for the Promotion of Science to 18K16582.
We thank Jane Charbonneau, DVM, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
References
1. AlMatter M, Bhogal P, Aguilar Pérez M, Hellstern V, Bäzner H, Ganslandt O. Evaluation of safety, efficacy and clinical outcome after endovascular treatment of aneurysmal subarachnoid hemorrhage in coil-first setting. A 10-year series from a single center. J Neuroradiol. 2018. 45: 349-56
2. Auer LM. Acute operation and preventive nimodipine improve outcome in patients with ruptured cerebral aneurysms. Neurosurgery. 1984. 15: 57-66
3. Ayling OG, Ibrahim GM, Drake B, Torner JC, Macdonald RL. Operative complications and differences in outcome after clipping and coiling of ruptured intracranial aneurysms. J Neurosurg. 2015. 123: 621-8
4. Batjer H, Samson D. Intraoperative aneurysmal rupture: Incidence, outcome, and suggestions for surgical management. Neurosurgery. 1986. 18: 701-7
5. Bechan RS, Sprengers ME, Majoie CB, Peluso JP, Sluzewski M, van Rooij WJ. Stent-assisted coil embolization of intracranial aneurysms: Complications in acutely ruptured versus unruptured aneurysms. AJNR Am J Neuroradiol. 2016. 37: 502-7
6. Berro DH, L’Allinec V, Pasco-Papon A, Emery E, Berro M, Barbier C. Clip-first policy versus coil-first policy for the exclusion of middle cerebral artery aneurysms. J Neurosurg. 2019. 133: 1-8
7. Billingsley JT, Hoh BL. Stent-assisted coil embolization. J Neurosurg. 2014. 121: 1-2
8. Brinjikji W, Piano M, Fang S, Pero G, Kallmes DF, Quilici L. Treatment of ruptured complex and large/giant ruptured cerebral aneurysms by acute coiling followed by staged flow diversion. J Neurosurg. 2016. 125: 120-7
9. Bulters DO, Santarius T, Chia HL, Parker RA, Trivedi R, Kirkpatrick PJ. Causes of neurological deficits following clipping of 200 consecutive ruptured aneurysms in patients with good-grade aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien). 2011. 153: 295-303
10. Edjlali M, Gentric JC, Régent-Rodriguez C, Trystram D, Hassen WB, Lion S. Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms?. Stroke. 2014. 45: 3704-6
11. Feng Z, Zuo Q, Yang P, Li Q, Zhao R, Hong B. Staged stenting with or without additional coils after conventional initial coiling of acute ruptured wide-neck intracranial aneurysms. World Neurosurg. 2017. 108: 506-12
12. Giannotta SL, Oppenheimer JH, Levy ML, Zelman V. Management of intraoperative rupture of aneurysm without hypotension. Neurosurgery. 1991. 28: 531-5
13. Houkin K, Kuroda S, Takahashi A, Takikawa S, Ishikawa T, Yoshimoto T. Intra-operative premature rupture of the cerebral aneurysms. Analysis of the causes and management. Acta Neurochir (Wien). 1999. 141: 1255-63
14. Inagawa T. Effect of early operation on cerebral vasospasm. Surg Neurol. 1990. 33: 239-46
15. Jartti P, Isokangas JM, Karttunen A, Jartti A, Haapea M, Koskelainen T. Early rebleeding after coiling of ruptured intracranial aneurysms. Acta Radiol. 2010. 51: 1043-9
16. Johnston SC, Dowd CF, Higashida RT, Lawton MT, Duckwiler GR, Gress DR. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: The cerebral aneurysm rerupture after treatment (CARAT) study. Stroke. 2008. 39: 120-5
17. Jomin M, Lesoin F, Lozes G. Prognosis with 500 ruptured and operated intracranial arterial aneurysms. Surg Neurol. 1984. 21: 13-8
18. Mascitelli JR, Lawton MT, Hendricks BK, Nakaji P, Zabramski JM, Spetzler RF. Analysis of wide-neck aneurysms in the barrow ruptured aneurysm trial. Neurosurgery. 2019. 85: 622-31
19. Matouk CC, Mandell DM, Günel M, Bulsara KR, Malhotra A, Hebert R. Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: Proof of principle. Neurosurgery. 2013. 72: 492-6
20. McLaughlin N, Bojanowski MW. Early surgery-related complications after aneurysm clip placement: An analysis of causes and patient outcomes. J Neurosurg. 2004. 101: 600-6
21. Mine B, Bonnet T, Vazquez-Suarez JC, Ligot N, Lubicz B. Evaluation of clinical and anatomical outcome of staged stenting after acute coiling of ruptured intracranial aneurysms. Interv Neuroradiol. 2020. 26: 260-7
22. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet. 2002. 360: 1267-74
23. Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet. 2015. 385: 691-7
24. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005. 366: 809-17
25. Nagahata S, Nagahata M, Obara M, Kondo R, Minagawa N, Sato S. Wall enhancement of the intracranial aneurysms revealed by magnetic resonance vessel wall imaging using three-dimensional turbo spin-echo sequence with motion-sensitized driven-equilibrium: A sign of ruptured aneurysm?. Clin Neuroradiol. 2016. 26: 277-83
26. Omodaka S, Endo H, Niizuma K, Fujimura M, Inoue T, Sato K. Quantitative assessment of circumferential enhancement along the wall of cerebral aneurysms using MR imaging. AJNR Am J Neuroradiol. 2016. 37: 1262-6
27. Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Nakaji P. Ten-year analysis of saccular aneurysms in the Barrow Ruptured Aneurysm Trial. J Neurosurg. 2019. 132: 771-6
28. Spetzler RF, Zabramski JM, McDougall CG, Albuquerque FC, Hills NK, Wallace RC. Analysis of saccular aneurysms in the barrow ruptured aneurysm trial. J Neurosurg. 2018. 128: 120-5
29. Steklacova A, Bradac O, de Lacy P, Lacman J, Charvat F, Benes V. “Coil mainly” policy in management of intracranial ACoA aneurysms: Single-centre experience with the systematic review of literature and meta-analysis. Neurosurg Rev. 2018. 41: 825-39
30. Waldau B, Reavey-Cantwell JF, Lawson MF, Jahshan S, Levy EI, Siddiqui AH. Intentional partial coiling dome protection of complex ruptured cerebral aneurysms prevents acute rebleeding and produces favorable clinical outcomes. Acta Neurochir (Wien). 2012. 154: 27-31