- Department of Neurosurgery, Saiseikai Fukuoka General Hospital, Chuo-ku, Fukuoka, Japan
- Department of Neurosurgery, Kurume University, School of Medicine, Kurume, Japan
- Department of Neurosurgery, Saiseikai Yahata General Hospital, Yahatahigashi-ku, Kitakyushu, Japan
- Department of Neurosurgery, Kumamoto University School of Medicine, Kumamoto, Japan
Correspondence Address:
Motohiro Morioka
Department of Neurosurgery, Kurume University, School of Medicine, Kurume, Japan
DOI:10.4103/sni.sni_18_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Akira Ohkura, Tetsuya Negoto, Takachika Aoki, Kei Noguchi, Yuji Okamoto, Hideki Komatani, Takayuki Kawano, Akitake Mukasa, Motohiro Morioka. Stenotic changes of the posterior cerebral artery are a major contributing factor for cerebral infarction in moyamoya disease. 24-May-2018;9:105
How to cite this URL: Akira Ohkura, Tetsuya Negoto, Takachika Aoki, Kei Noguchi, Yuji Okamoto, Hideki Komatani, Takayuki Kawano, Akitake Mukasa, Motohiro Morioka. Stenotic changes of the posterior cerebral artery are a major contributing factor for cerebral infarction in moyamoya disease. 24-May-2018;9:105. Available from: http://surgicalneurologyint.com/surgicalint-articles/stenotic-changes-of-the-posterior-cerebral-artery-are-a-major-contributing-factor-for-cerebral-infarction-in-moyamoya-disease/
Abstract
Background:Some patients with moyamoya disease (MMD) show broad infarction with moderate internal carotid artery (ICA) stenosis, whereas others with complete ICA occlusion show no infarction. This suggests that other factors contribute to the occurrence of infarction. Contributing factors predictive of cerebral infarcts must be identified for the prevention of infarction and the consequent neurological deficits.
Methods:We examined data from 93 patients with confirmed MMD for the presence of infarction (n = 72), transient ischemic attack (TIA, n = 41), asymptomatic presentation (n = 51), or hemorrhage (n = 22) in 186 bilateral cerebral hemispheres. We analyzed the relationship between the occurrence of infarction and several clinical factors, such as steno-occlusive status or the site of the ICA and posterior cerebral artery (PCA).
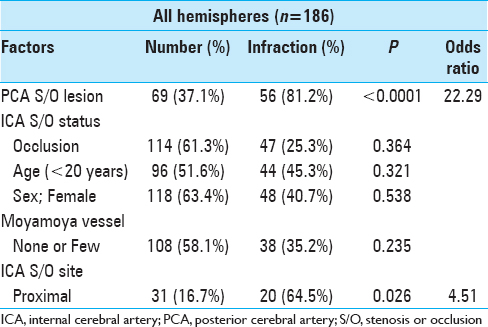
Results:The incidence of PCA steno-occlusive lesions was significantly higher in infarcted (77.8%) than in non-infarcted hemispheres (TIA, 14.6%; asymptomatic, 9.8%; hemorrhagic 9.1%; P P P
Conclusions:This is the multivariate statistical analysis study identifying PCA steno-occlusive lesions as the most important independent factor that is predictive to cerebral infarction in moyamoya patients. The prediction and inhibition of PCA steno-occlusive changes may help to prevent cerebral infarction.
Keywords: Cerebral infarction, moyamoya disease, multivariate analysis, posterior cerebral artery, transient ischemic attacks
INTRODUCTION
Moyamoya disease (MMD) is an unusual form of chronic, occlusive cerebrovascular disease characterized by bilateral stenosis or occlusion at the terminal portion of the internal carotid artery (ICA) and an abnormal vascular network (the so-called moyamoya vessels) at the base of the brain.[
Although bypass surgery improves the prognosis of moyamoya patients with cerebral infarction or transient ischemic attacks (TIA),[
As cerebral infarction may produce serious neurological deficits, it is important to identify factors predictive of its occurrence. Our previous experience with pediatric MMD patients suggested that steno-occlusive changes in the posterior cerebral artery (PCA) can be implicated in progression to cerebral infarction,[
MATERIALS AND METHODS
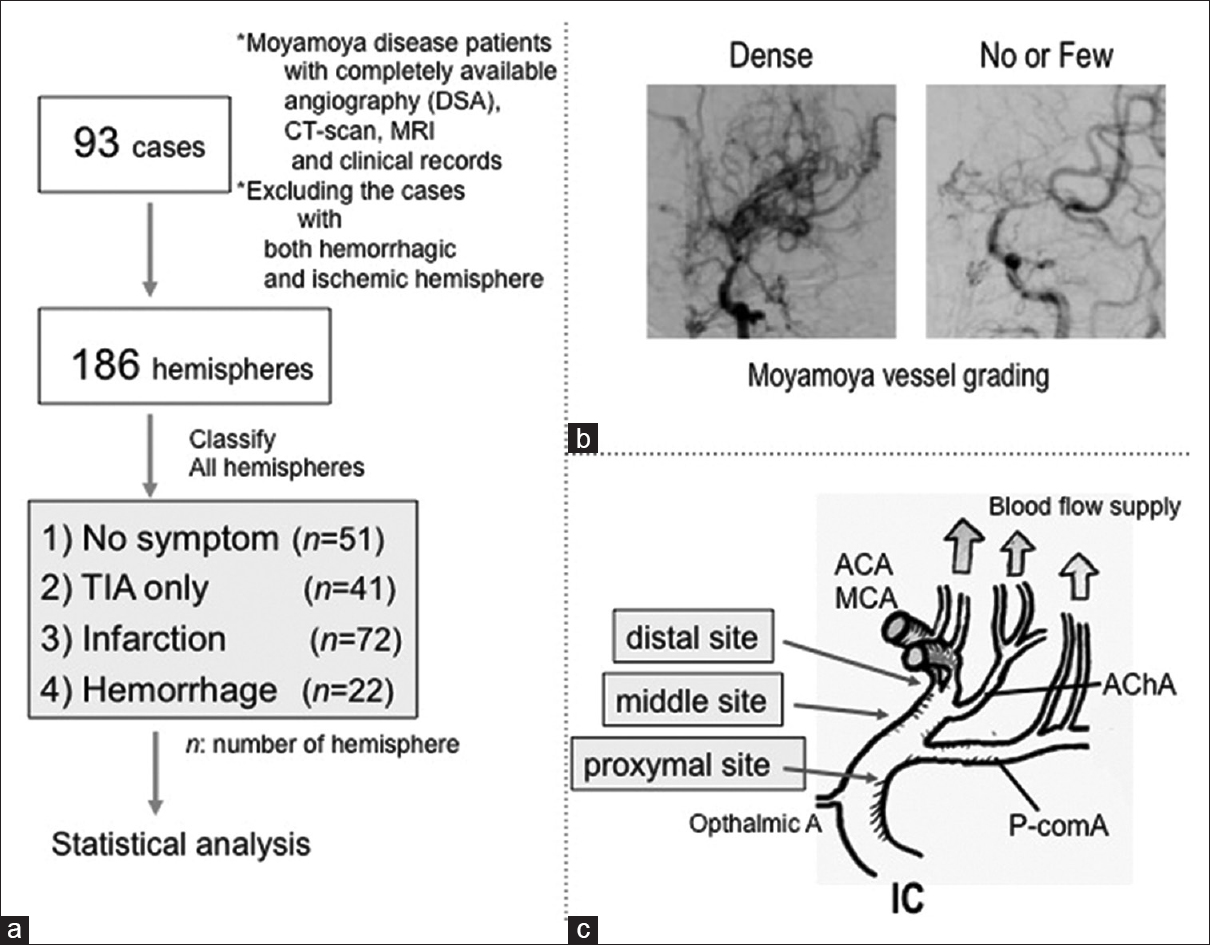
We obtained detailed clinical records of 93 MMD patients treated between January 2008 and December 2015 at the Departments of Neurosurgery of Kumamoto University and Kurume University [
Figure 1
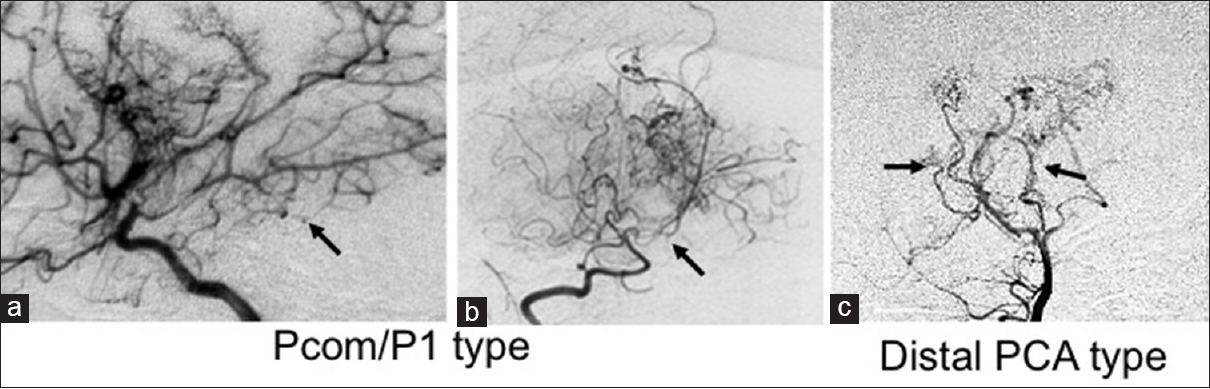
(a) Flowchart of statistical analysis study data. (b) Grading of the moyamoya vessels as “Dense” and “No or Few,” according to a previous report by Morioka et al., 2003.[
Both cerebral hemispheres of the 93 patients (n = 186) were separately assessed as asymptomatic or manifesting TIA, infarction, or hemorrhage [
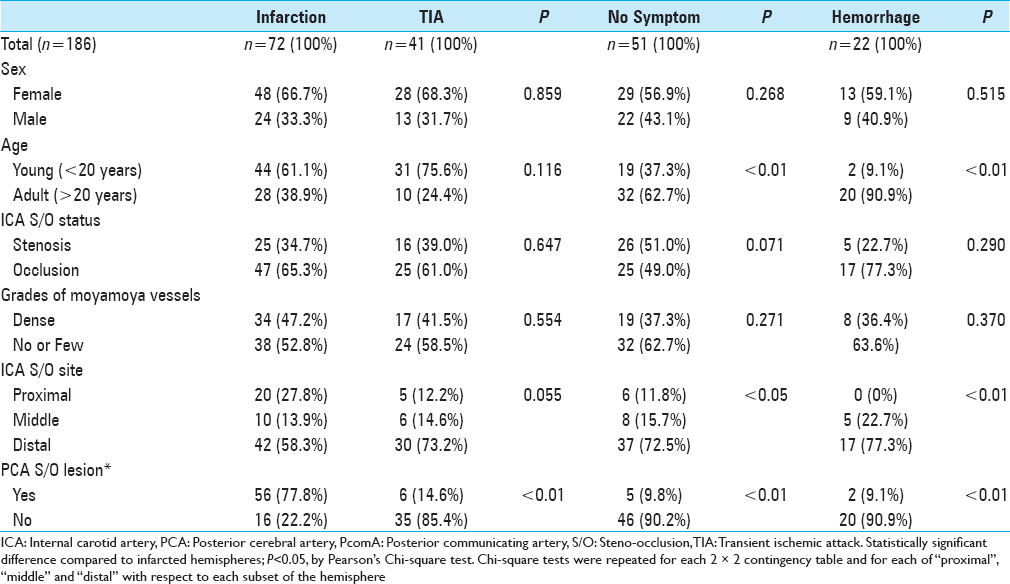
PCA steno-occlusive status was defined as “No” or “Yes,” as evaluated using both, carotid and vertebral angiographies. PCA steno-occlusive sites were classified into two categories as 1) PCA-P1 type (proximal to PCA P1 portion including PcomA)[Figure
Figure 2
Patterns of PcomA/PCA stenosis (a-c) (1) PcomA/P1 type: (A) right ICA angiograph (lateral view) showing PcomA and PCA stenosis. (b) Right vertebral artery angiograph (VAG, antero-posterior view) demonstrating P1 occlusion; (2) Distal PCA type: (c) VAG (antero-posterior view) shows both distal PCA stenosis
Statistical analysis to determine the possibility of clinical data to contribute to infarction occurrence was carried out with Pearson's Chi-square test. Chi-square tests were repeated for each 2 × 2 contingency table and for each of the “proximal,” “middle,” and “distal” regions of each subset of each hemisphere. Multivariate analysis was conducted using multiple logistic-regression analysis. Differences with P < 0.01 and P < 0.05 were considered statistically significant.
RESULTS
The mean age of the 74 MMD patients with ischemic symptoms (48 females and 26 males) was 19.9 (17.7) years (mean [SD]), ranging from 2.2 to 67.7 years. The 19 patients with hemorrhagic symptoms (11 females and 8 males) ranged in age from 5.3 to 59.4 (mean 39.6 [14.8]) years.
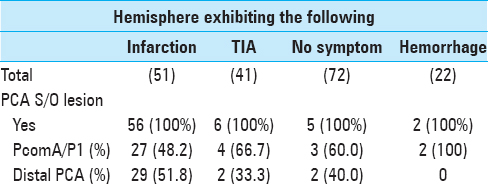
First, we looked for factors that may have contributed to the occurrence of infarction by comparing the infarction-hemisphere (n = 72) with TIA (n = 41), hemorrhage (n = 22), and no symptom (n = 51) hemispheres. There was no significant difference in the sex, ICA steno-occlusive status, or grade of moyamoya vessels.
The ICA steno-occlusive site in 20 of the 72 infarcted hemispheres (27.8%) was proximal, that is, proximal to the PcomA [
Although the hemispheres of the younger patients (under 20 years of age) tended to display ischemic symptoms (TIA or infarction), there was no obvious significant difference between the infarcted and TIA hemispheres.
Subsequently, we examined the frequency and sites of steno-occlusive lesions in the PCA, which were detected at PCA-P1 type [Figure
Using multiple logistic-regression analysis, we performed a multivariate analysis of the association between several factors, such as young age of patient (<20 years), female sex of patient, no or few grades of moyamoya vessels, PCA steno-occlusive lesions, ICA occlusion lesions, and ICA steno-occlusive lesion at the proximal side [
DISCUSSION
We detected PCA steno-occlusive lesions in 69 (37.1%) of the 186 hemispheres from patients with MMD. This incidence is in concordance with the rate reported by others.[
Relationship between PCA and the ICA steno-occlusive site
Mugikura et al.[
Mechanism of the development of infarction in moyamoya disease
Based on our data, we developed a hypothesis regarding the mechanism underlying the development of infarction in patients with MMD. In this disease, the cerebral blood flow to the anterior circulation territory is delivered through four routes: (a) the stenotic ICA/ACA/MCA, (b) moyamoya vessels, (c) perforators of the AChA/PcomA[
Although this study demonstrated that a PCA steno-occlusive change is an obvious contributing factor to infarction, the lesions were not detected in 16 of the 72 infarcted hemispheres. Although the mechanism underlying this type of infarction is not well understood, we posit that stenotic ICA/ACA/MCA changes can progress rapidly without sufficient collateral flow provided by the moyamoya or other vessels.
Distinction between infarction and TIA in moyamoya disease
Although TIA and infarction are considered ischemic symptoms in patients with MMD, TIA and infarcted hemispheres have obviously different characteristics and exhibit clearly differing prognoses. We distinguished the ischemic symptoms between TIA and infarction in our study, and found a significant difference between these in cases with ICA proximal steno-occlusive lesions and PCA steno-occlusive lesions. According to our data, TIA-hemisphere with sufficient PCA flow has a lower risk of infarction. Thus, frequent TIA symptoms may not be related to infarction. Although the pathogenesis of MMD remains largely unknown, inhibiting the progression of stenosis-occlusion in the PCA may reduce the incidence of cerebral infarction in these patients.
Study limitations
The study was designed based on angiographic assessment and thus did not include quantitative or semi-quantitative grading. Furthermore, we could not obtain data from (semi-) quantitative analysis by SPECT or PET because of institutional differences in evaluation. However, we consider that angiographic assessment is sufficient to assess the extent of the collateral development.
CONCLUSIONS
This multivariate statistical analysis study identifies PCA steno-occlusive lesions as the most important independent factor for prediction of cerebral infarction in moyamoya patients. This prediction and inhibition of PCA steno-occlusive changes may help prevent cerebral infarction in these patients. Moreover, we found that the proximal site of the ICA steno-occlusive lesion had a major effect on PCA flow-related brain infarction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Funaki T, Takahashi JC, Takagi Y, Yoshida K, Araki Y, Kikuchi T. Impact of posterior cerebral artery involvement on long-term clinical and social outcome of pediatric moyamoya disease. J Neurosurg Pediatr. 2013. 12: 626-32
2. Ge P, Zhang Q, Ye X, Liu X, Deng X, Wang R. Clinical features, surgical treatment, and long-term outcome in elderly patients with moyamoya disease. World Neurosurg. 2017. 100: 459-66
3. Hishikawa T, Tokunaga K, Sugiu K, Date I. Assessment of the difference in posterior circulation involvement between pediatric and adult patients with moyamoya disease. J Neurosurg. 2013. 119: 961-5
4. Hishikawa T, Tokunaga K, Sugiu K, Date I. Long-term outcomes in adult patients with ischemic-type moyamoya disease involving posterior circulation. Acta Neurochir (Wien). 2014. 156: 1745-51
5. Ji YL, Seung KK, Ji HP, Kyu CW. Posterior cerebral artery insufficiency in pediatric moyamoya disease. J Korean Neurosurg Soc. 2015. 57: 436-9
6. Karasawa J, Kikuchi H, Furuse S, Kawamura J, Sakaki T. Treatment of moyamoya disease with STA-MCA anastomosis. J Neurosurg. 1978. 49: 679-88
7. Karasawa J, Touho H, Ohnishi H, Miyamoto S, Kikuchi H. Long-term follow-up study after extracranial-intracranial bypass surgery for anterior circulation ischemia in childhood moyamoya disease. J Neurosurg. 1992. 77: 84-9
8. Kuroda S, Ishikawa T, Houkin K, Iwasaki Y. Clinical significance of posterior cerebral artery stenosis/occlusion in moyamoya disease. No Shinkei Geka. 2002. 30: 1295-300
9. Masuda J, Ogata J, Yamaguchi T, Barnett HJM, Mohr JP, Stein BM, Yatsu FM.editors. Moyamoya disease. Stroke, Pathophysiology, Diagnosis, and Management. Churchill Livingston: New York: 1998. p. 815-32
10. Matsushima Y, Fukai N, Tanaka K, Tsuruoka S, Inaba Y, Aoyagi M. A new surgical treatment of moyamoya disease in children: A preliminary report. Surg Neurol. 1981. 15: 313-20
11. Miyamoto S, Kikuchi H, Karasawa J, Nagata I, Ikota T, Takeuchi S. Study of the posterior circulation in moyamoya disease: Clinical and neuroradiological evaluation. J Neurosurg. 1984. 61: 1032-37
12. Morioka M, Hamada J, Kawano T, Todaka T, Yano S, Kai Y, Ushio Y. The angiographical dilatation and branch extension of the anterior choroidal and posterior communicating artery are predictors of hemorrhage in adult moyamoya patients. Stroke. 2003. 34: 90-5
13. Morioka M, Hamada J, Todaka T, Yano S, Kai Y, Ushio Y. High-risk age for rebleeding in patients with hemorrhagic moyamoya disease: Long-term follow-up study. Neurosurgery. 2003. 52: 1049-55
14. Morioka M, Hamada J, Kai Y, Yano S, Kawano T, Ohmori Y. Contributing factors to long-term outcome and type of onset in young aged moyamoya disease patients with ischemic onset. Surg Cereb Stroke. 2009. 37: 338-44
15. Mugikura S, Takahashi S, Higano S, Shirane R, Kurihara N, Furuta S. The relationship between cerebral infarction and angiographic characteristics in childhood moyamoya disease. Am J Neuroradiol. 1999. 20: 336-43
16. Mugikura S, Takahashi S, Higano S, Shirane R, Sakurai Y, Yamada S. Predominant involvement of ipsilateral anterior and posterior circulations in moyamoya disease. Stroke. 2002. 33: 1497-500
17. Mugikura S, Higano S, Shirane R, Fujimura M, Shimanuki Y, Takahashi S. Posterior circulation and high prevalence of ischemic stroke among young pediatric patients with moyamoya disease: Evidence of angiography-based differences by age at diagnosis. Am J Neuroradiol. 2011. 32: 192-8
18. Nishimoto A, Takeuchi S. Abnormal cerebrovascular network related to the internal carotid arteries. J Neurosurg. 1968. 29: 255-60
19. Suzuki J, Kodama N. Moyamoya disease-A review. Stroke. 1983. 14: 104-9
20. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969. 20: 288-99
21. Yamada I, Himeno Y, Suzuki S, Matsushima Y. Posterior circulation in moyamoya disease: Angiographic study. Radiology. 1995. 197: 239-46