- Department of Neurosurgery, Kita-Harima Medical Center, Ono, Japan
- Department of Neurosurgery, Kobe University Graduate School of Medicine, Kobe, Japan.
Correspondence Address:
Ayaka Shibano, Department of Neurosurgery, Kita-Harima Medical Center, Ono, Japan.
DOI:10.25259/SNI_619_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ayaka Shibano1, Junichi Sakata1, Atsushi Fujita2, Tomoaki Harada1, Reiichi Okino1, Daisuke Yamamoto1, Shigeru Miyake1. Stent assisted coil embolization for a dissecting cerebral aneurysm of middle cerebral artery: A case report and the literature review. 20-Oct-2023;14:375
How to cite this URL: Ayaka Shibano1, Junichi Sakata1, Atsushi Fujita2, Tomoaki Harada1, Reiichi Okino1, Daisuke Yamamoto1, Shigeru Miyake1. Stent assisted coil embolization for a dissecting cerebral aneurysm of middle cerebral artery: A case report and the literature review. 20-Oct-2023;14:375. Available from: https://surgicalneurologyint.com/surgicalint-articles/12608/
Abstract
Background: Dissecting aneurysms of the middle cerebral artery (MCA) are very rare. We herein report a case of an unruptured dissecting aneurysm of the MCA treated by stent-assisted coil embolization.
Case Description: A 65-year-old man with no history of trauma presented with a headache. Time-of-flight imaging revealed a dissecting cerebral aneurysm in the right M1 segment of the MCA, and the aneurysm had increased in size within a short time. We treated the aneurysm by endovascular stenting with coils, and the patient developed no neurological deficits.
Conclusion: Because of the potential involvement of the lenticulostriate artery (LSA) in the area of dissection, choosing the best treatment (such as direct surgery or endovascular treatment) may be challenging. Treatment efficacy depends on whether the LSA is affected and on the length of the dissection. In our case, the dissection did not involve the LSA and could therefore be treated by stent-assisted coil embolization.
Keywords: Dissecting aneurysm, Middle cerebral artery, Stent-assisted coil embolization
INTRODUCTION
Intracranial dissecting aneurysms are rare clinical entities. Most intracranial dissecting aneurysms occur in the vertebrobasilar artery. Dissecting aneurysms in the internal carotid artery (ICA) is uncommon (12.6%).[
CASE REPORT
A 65-year-old man with no history of trauma developed a headache and presented to the outpatient neurosurgery clinic. He was referred to our department because of a dissecting cerebral aneurysm in the right M1 segment of the MCA [
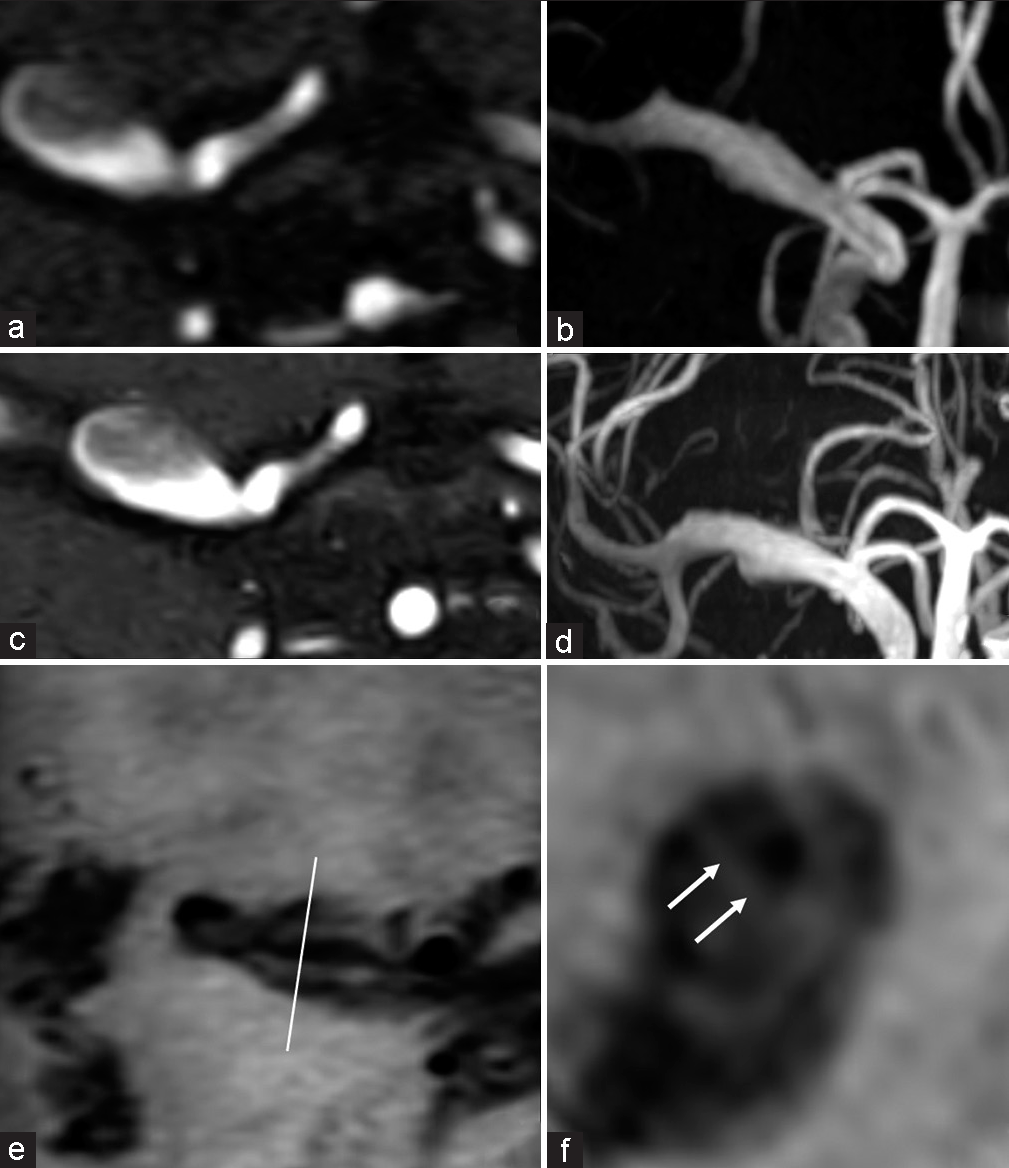
Figure 1:
Initial magnetic resonance angiography at presentation. (a) Source imaging, (b) maximum intensity projection showed an enlarged middle cerebral artery, (c, d) 10 days after the onset of the headache, these images showed enlargement of the M1 segment, (e) axial T1-weighted black-blood imaging, and (f) multiplanar imaging showed a double lumen (arrows).
Endovascular procedure
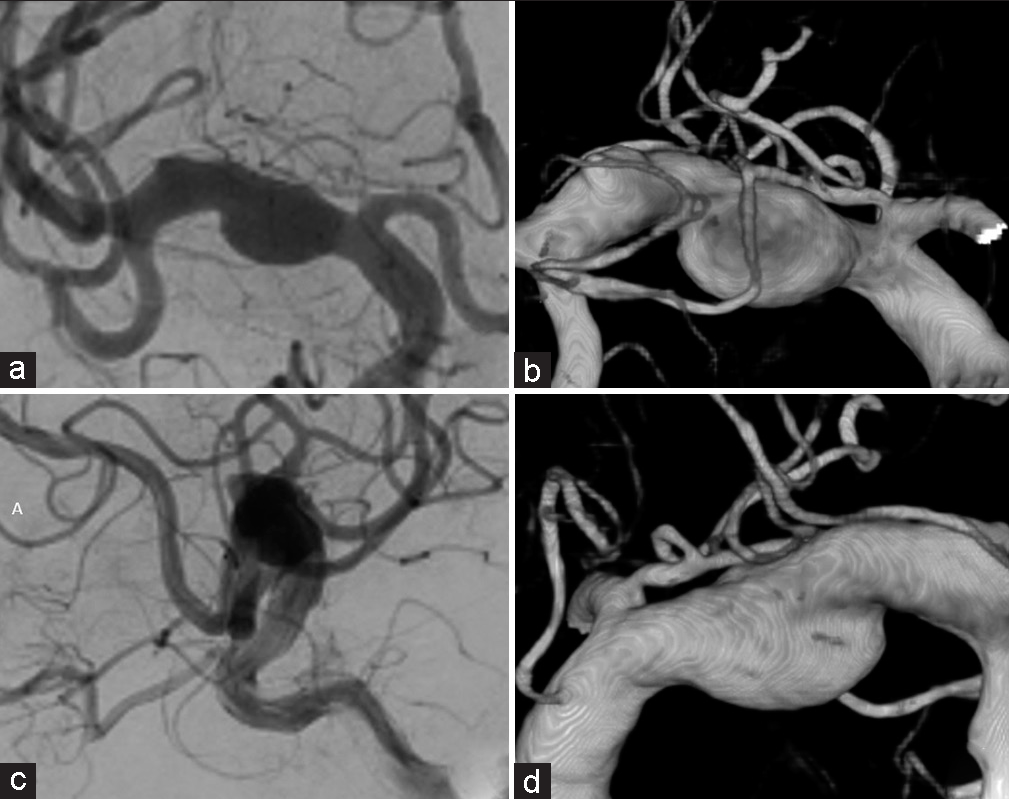
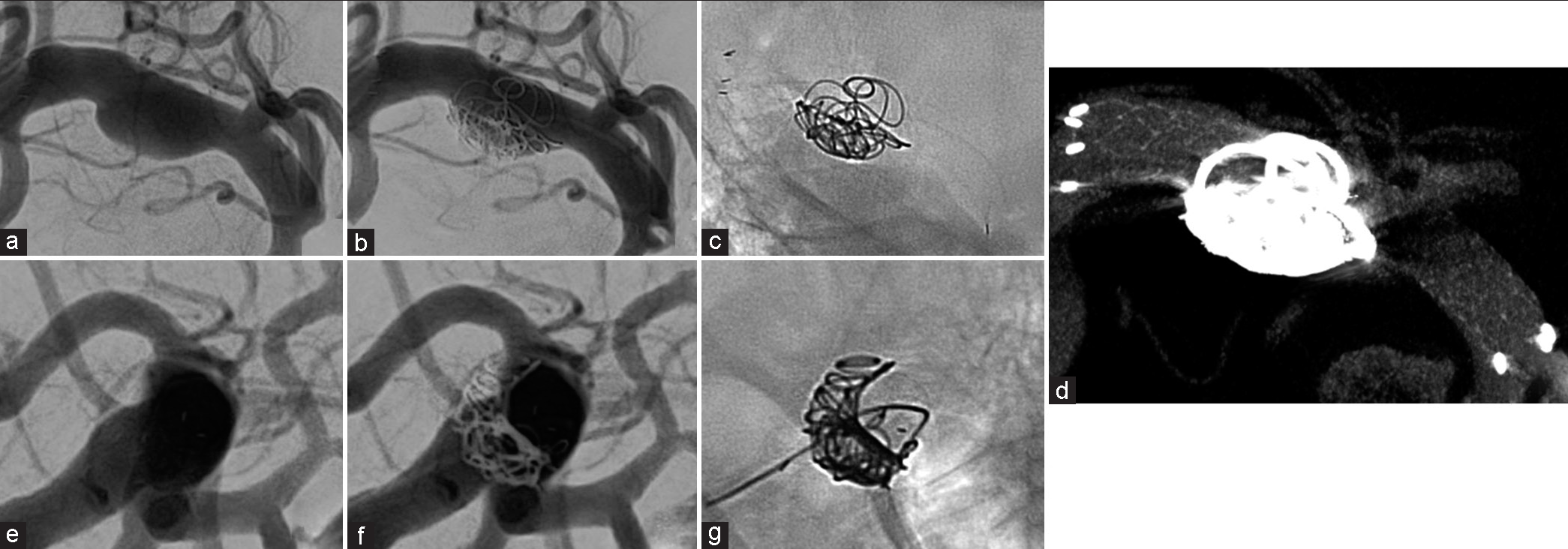
The endovascular therapy was performed 17 days after onset. Dual antiplatelet therapy (aspirin 100 mg and clopidogrel 75 mg) was started 4 days preoperatively. With the patient under general anesthesia, a 9-French (Fr) introducer was placed in the right femoral artery. After systemic heparization, a 9-Fr guiding catheter (Optimo; Tokai Medical Products, Aichi, Japan) was placed in the right common carotid artery, and a 6-Fr distal access catheter (Cerulean DD6 Plus; Medikit, Tokyo, Japan) was placed in the ICA. An ICA angiogram confirmed the increase in the size of the dissecting cerebral aneurysm. A stent delivery microcatheter (Prowler Select Plus; Johnson and Johnson, New Brunswick, NJ, USA) was advanced distally to the dissecting lesion M2 segment, and a coil delivery microcatheter (SL-10; Stryker Neurovascular, Fremont, CA, USA) was then placed in the aneurysm. A 4-mm × 39-mm stent (Enterprise 2 VRD; Codman, Raynham, MA, USA) was deployed from the distal end of the M1 segment to the ICA. Two detachable coils (iED ∞3–5 mm × 20 cm, i-ED ∞3–5 mm × 15 cm; Kaneka Corp., Osaka, Japan) were inserted into the aneurysm [
Postoperative course
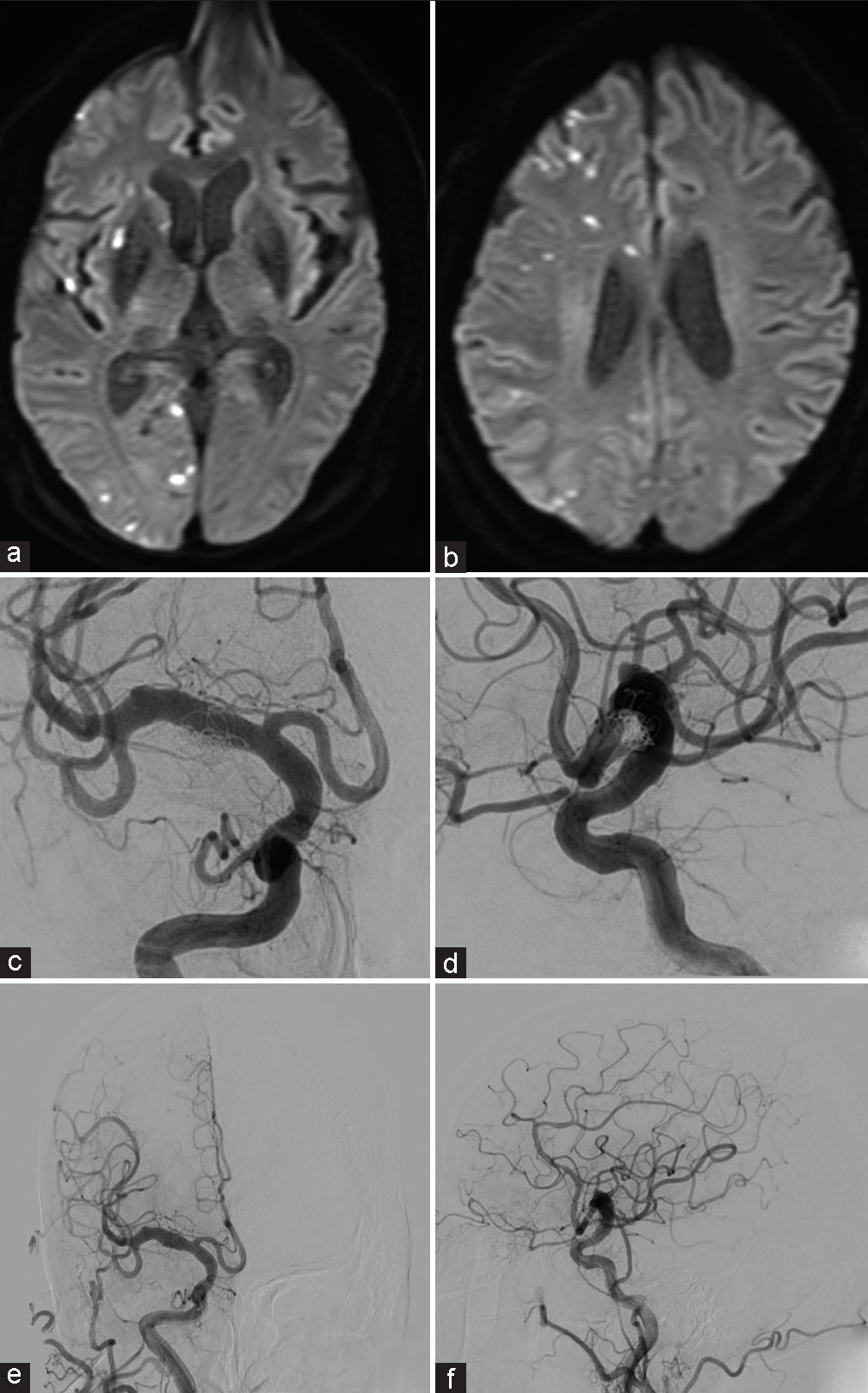
On the 1st postoperative day, diffusion-weighted imaging revealed sporadic high-intensity signals in the right cerebral hemisphere [
Figure 4:
(a and b) Postoperative diffusion-weighted imaging revealed sporadic areas of high signal intensity in the right cerebral hemisphere, (c and d) digital subtraction angiograms obtained 3 days after treatment showed complete occlusion of the dissecting lumen and good remodeling of the M1 segment. (e and f) Four-month follow-up angiograms showed complete obliteration of the dissecting aneurysm with preservation of the M1 segment.
DISCUSSION
Most intracranial dissecting aneurysms are located in the vertebrobasilar artery (87.4%); and dissecting aneurysms in the ICA are uncommon (12.6%).[
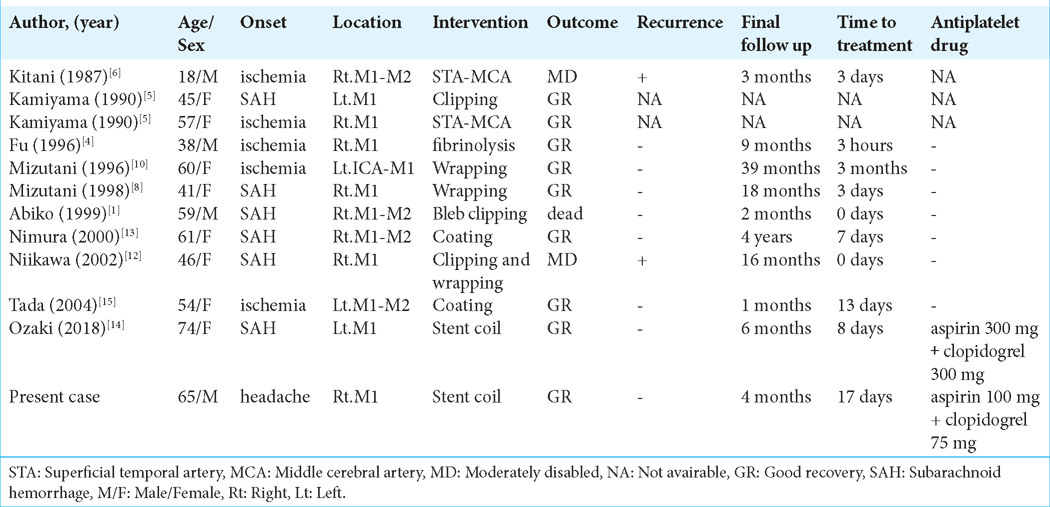
Dissecting aneurysms of the MCA are very rare and have been described in only 11 case reports.[
Treatment of an MCA dissecting aneurysm is challenging because the lesion may involve the LSA. There are few reports on the surgical or endovascular treatment of MCA dissecting aneurysms. Because of the presence of perforating arteries, wrapping is often performed for dissecting aneurysms of the M1 segment and the M1–M2 junction. However, the risk of rupture remains. Dissecting aneurysms of the M2 segment are treated with bypass and trapping because there are fewer perforators from the M2 segment.[
Dissecting cerebral aneurysms of the M1 segment of the MCA is particularly difficult to treat surgically because of the potential presence of the LSA in the dissecting lesion. Treatment efficacy depends on whether the LSA is affected and on the length of the dissection.
In our case, the LSA was contralateral to the dissection, and we were thus able to preserve it after stent-assisted coil embolization.
The occlusion rate of craniotomy clipping surgery is 94.2%, and the occlusion rate of simple coil technique is 54.2%, while the occlusion rate of flow diverter (FD) is about 80%, which is a good result. A meta-analysis of FD treatment for 244 MCA aneurysms reported 20.7% complications and 10% reported permanent neurological sequelae. Unlike the favorable results of FD treatment for aneurysms in the anterior circulation, many of them have been reported to be due to perforator ischemia covered by FD, and they concluded that caution is required in its indication.[
CONCLUSION
We have reported a case of an unruptured dissecting aneurysm of the MCA that was successfully treated by stent-assisted coil embolization. The best treatment option for this condition is difficult to determine, and an accurate assessment of imaging findings is crucial.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abiko S, Okamura T, Kurokawa Y, Ikeda N, Ideguchi M, Watanabe K. Diagnosis and treatment of nontraumatic dissecting aneurysm in the middle cerebral artery. No Shinkei Geka. 1999. 27: 743-9
2. Cagnazzo F, Mantilla D, Lefevre PH, Dargazanli C, Gascou G, Costalat V. Treatment of middle cerebral artery aneurysms with flow-diverter stents: A systemic review and meta-analysis. AJNR Am J Neuroradiol. 2017. 38: 2289-94
3. Day AL, Gaposchkin CG, Yu CJ, Rivet DJ, Dacey RG. Spontaneous fusiform middle cerebral artery aneurysms: Characteristics and a proposed mechanism of formation. J Neurosurg. 2003. 99: 228-40
4. Fu Y, Komiyama M, Inoue T, Ohata K, Matsuoka Y, Hakuba A. A case of middle cerebral artery occlusion caused by dissecting aneurysm. No Shinkei Geka. 1996. 24: 955-9
5. Kamiyama H, Nomura M, Abe H, Abumiya T, Saito H, Yamauchi T. Diagnosis for the intracranial dissecting aneurysms. Surg Cereb Stroke. 1990. 18: 50-6
6. Kitani R, Itouji T, Noda Y, Kimura M, Uchida S. Dissecting aneurysms of the anterior circle of willis arteries. Report of two cases. J Neurosurg. 1987. 67: 296-300
7. Mizutani T. Natural course of intracranial arterial dissections. J Neurosurg. 2011. 114: 1037-44
8. Mizutani T. Surgical considerations for cerebral dissecting aneurysms. Surg Cereb Stroke. 2002. 30: 424-8
9. Mizutani T. Middle cerebral artery dissecting aneurysm with persistent patent pseudolumen. J Neurosurg. 1996. 84: 267-8
10. Mizutani T. Subarachnoid hemorrhage associated with angiographic “stenosis” or “occlusive” lesions in the carotid circulation. Surg Neurol. 1998. 49: 495-504
11. Nam DH, Park SK. Endovascular treatment in ruptured middle cerebral artery dissection preservation of arterial continuity. J Cerebrovasc Endovasc Neurosurg. 2015. 17: 108-12
12. Niikawa S, Yamada J, Sumi J, Yamakawa H. Dissecting aneurysm of middle cerebral artery manifesting as subarachnoid hemorrhage and hemorrhagic infarctions. Case report. Neurol Med Chir(Tokyo). 2002. 42: 62-6
13. Nimura T, Oku T, Narita N, Higuchi H. A case of middle cerebral artery dissecting aneurysm. No Shinkei Geka. 2000. 28: 61-5
14. Ozaki T, Nishida T, Fujita Y, Kishima H, Kinoshita M. Coil and single-stent placement for ruptured dissecting aneurysm of middle cerebral artery: A case report. World Neurosurg. 2018. 113: 208-11
15. Tada Y, Kannuki S, Koyama Y. Dissecting aneurysm of the middle cerebral artery growing during long-term follow-up: A case report. Surg Cereb Stroke. 2004. 32: 370-5
16. Zhao T, Zhu D, Wen W, Zhuo Y, Fang Y, Li Q. Endovascular treatment of middle cerebral artery dissecting aneurysms: A 7-year single-center study. World neurosurgery. 2018. 112: 119-24