- Department of Neurosurgery, National Hospital Organization Osaka National Hospital, Chuoku, Osaka, Japan.
- Central Laboratory and Surgical Pathology, National Hospital Organization Osaka National Hospital, Chuoku, Osaka, Japan.
Correspondence Address:
Tomohiko Ozaki, Department of Neurosurgery, National Hospital Organization Osaka National Hospital, Chuoku, Osaka, Japan.
DOI:10.25259/SNI_1126_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Naoki Nishizawa1, Tomohiko Ozaki1, Tomoki Kidani1, Shin Nakajima1, Yonehiro Kanemura1, Keisuke Nishimoto1, Hiroki Yamazaki1, Kiyoshi Mori2, Toshiyuki Fujinaka1. Stent infection and pseudoaneurysm formation after carotid artery stent treated by excision and in situ reconstruction with polytetrafluoroethylene graft: A case report. 20-Jan-2022;13:24
How to cite this URL: Naoki Nishizawa1, Tomohiko Ozaki1, Tomoki Kidani1, Shin Nakajima1, Yonehiro Kanemura1, Keisuke Nishimoto1, Hiroki Yamazaki1, Kiyoshi Mori2, Toshiyuki Fujinaka1. Stent infection and pseudoaneurysm formation after carotid artery stent treated by excision and in situ reconstruction with polytetrafluoroethylene graft: A case report. 20-Jan-2022;13:24. Available from: https://surgicalneurologyint.com/surgicalint-articles/11352/
Abstract
Background: Stent infection after carotid artery stenting (CAS) can be a life-threatening postoperative complication, but there is a paucity of data due to its exceedingly low frequency. We report a case of stent infection with pseudoaneurysm formation after CAS that was treated through replacing the infected stent and pseudoaneurysm with a polytetrafluoroethylene (PTFE) synthetic vessel graft.
Case Description: An 86-year-old man was treated for the right internal carotid artery with CAS in local hospital. One month after stenting, he suffered aspiration pneumonia and septicemia. Three months after stenting, swelling and tenderness of the right side of his neck appeared. His general condition deteriorated due to septicemia and he was unable to ingest anything by mouth as a result of decreasing levels of consciousness. He was transferred to our hospital. Computed tomography and digital subtraction angiography showed the presence of a pseudoaneurysm around the stent. The neck mass enlarged daily and surgical intervention was required to prevent closure of the airway. Stent and pseudoaneurysm resection and in situ reconstruction with a PTFE synthetic vessel graft were performed. The patient returned to his local hospital 36 days after surgery and had a modified Rankin Score of 5.
Conclusion: Although the risk of reinfection is high due to the nature of artificial material, stent/pseudoaneurysm resection and in situ reconstruction with a PTFE synthetic vessel graft might be one of the best options for patients suffering stent infection after CAS. To the best of our knowledge, this is the first report of treatment using this material.
Keywords: In situ reconstruction with polytetrafluoroethylene graft, Pseudoaneurysm formation after carotid artery stent, Stent infection
INTRODUCTION
Stent infection after carotid artery stenting (CAS) is an extremely rare complication and there is no consensus regarding treatment despite high mortality rates. These infections often cause arterial destruction and pseudoaneurysm formation.[
CASE PRESENTATION
An 86-year-old man was treated for symptomatic (transient left hemiparesis) right internal carotid artery (ICA) with CAS in his local hospital. One month after stenting, he suffered aspiration pneumonia and septicemia, and Klebsiella oxytoca was isolated on blood culture. Three months after stenting, swelling and tenderness of the right side of his neck appeared. His general condition deteriorated due to septicemia and he was unable to ingest anything by mouth because of his decreasing level of consciousness. Five months after stenting, he was transferred to our hospital [
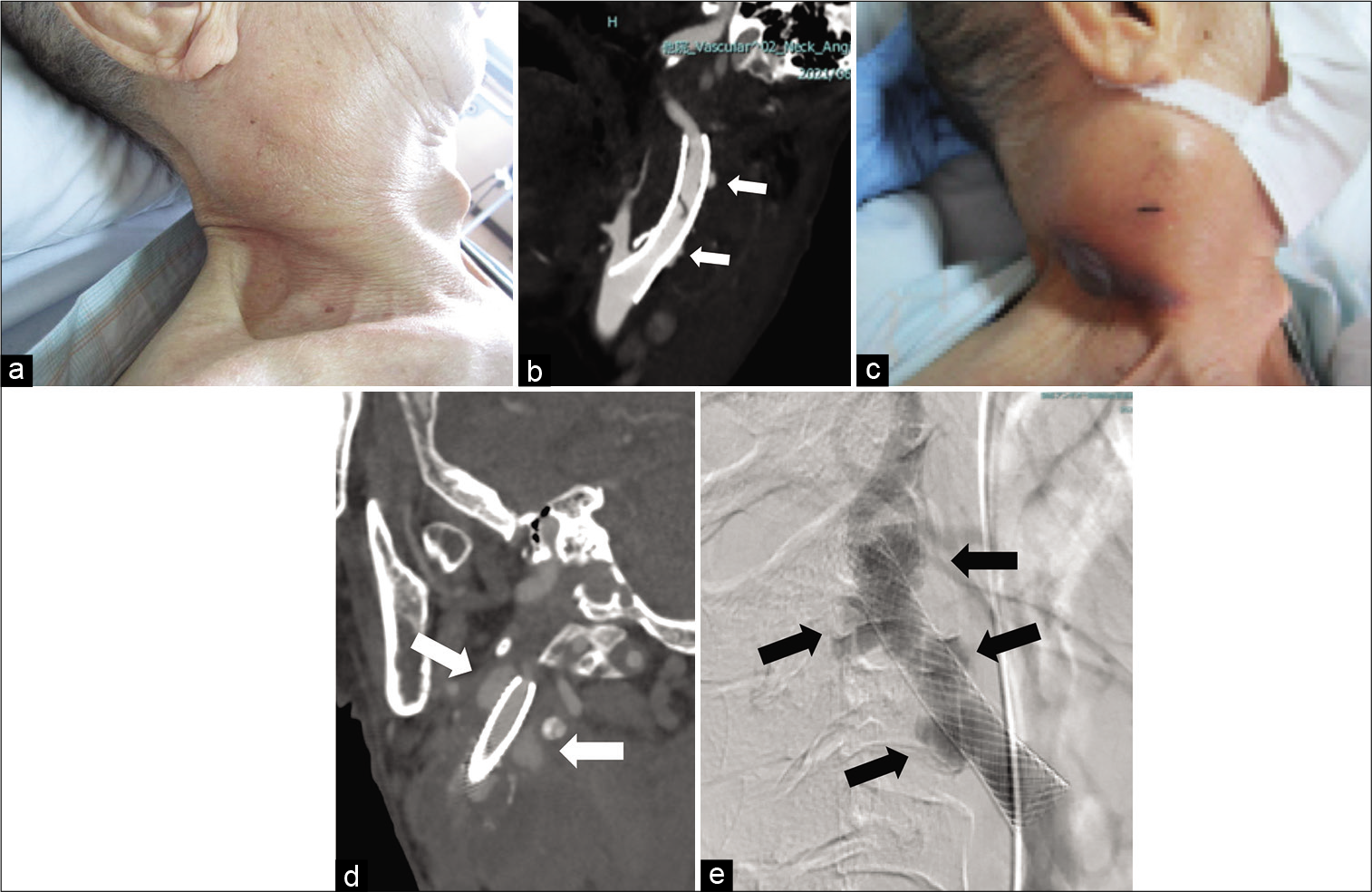
Figure 1:
Clinical images before surgery. (a) Picture taken on the day the patient was transferred to our hospital showing swelling of the right side of the neck. (b) CTA taken the day the patient was transferred to our hospital showing the stent placed in the right ICA-CCA and contrast material outside the stent (arrow). (c) Picture taken 2 weeks after admission showing growth of the mass. (d) CTA taken 2 weeks after admission showing an increase in the size of the region that contrast material was flowing into outside the stent (arrow). (e) DSA showing contrast material flowing outside the stent (arrow). CTA: Computed tomography angiography, ICA: Internal carotid artery, CCA: Common carotid artery, DSA: Digital subtraction angiography.
The operation was performed under general anesthesia. A skin incision was made over the anterior border of the sternocleidomastoid muscle extending to the root of the zygoma. First, the common carotid artery (CCA) proximal to the stent was secured. Then, the styloid process and mandibular angle distal to the stent and pseudoaneurysm were cut to secure the ICA [
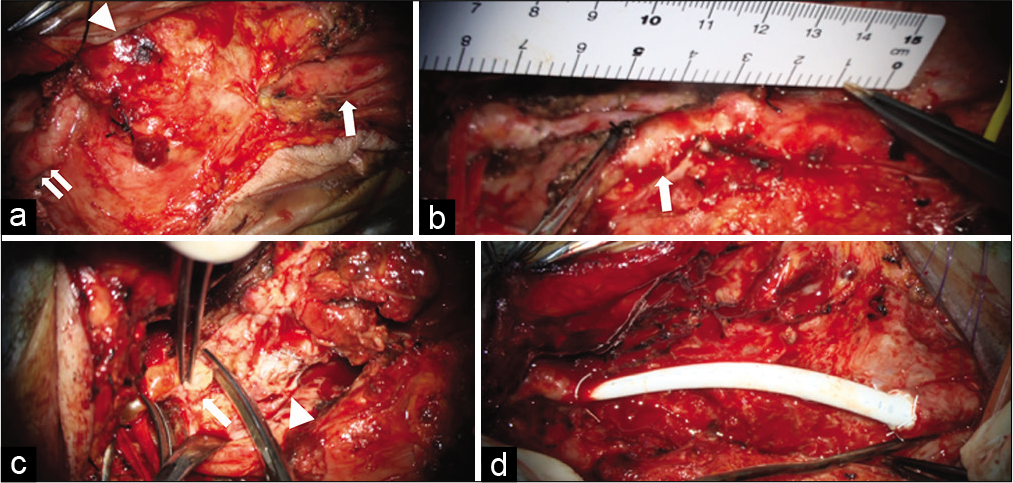
Figure 2:
Perioperative pictures. (a) Pseudoaneurysm (arrowhead) and the normal region of the CCA (arrow) and ICA (double arrow). (b) ECA (arrow) was cut at the distal location of the pseudoaneurysm. (c) ICA distal to pseudoaneurysm. Arrow shows normal intima and arrowhead shows the stent inside the ICA. (d) PTFE synthetic vessel graft reconstruction with continuous suture of CV-5 Gore-Tex. CCA: Common carotid artery, ICA: Internal carotid artery, ECA: External carotid artery, PTFE: Polytetrafluoroethylene.
Histopathological investigation showed rupture of the arterial wall and formation of a pseudoaneurysm [
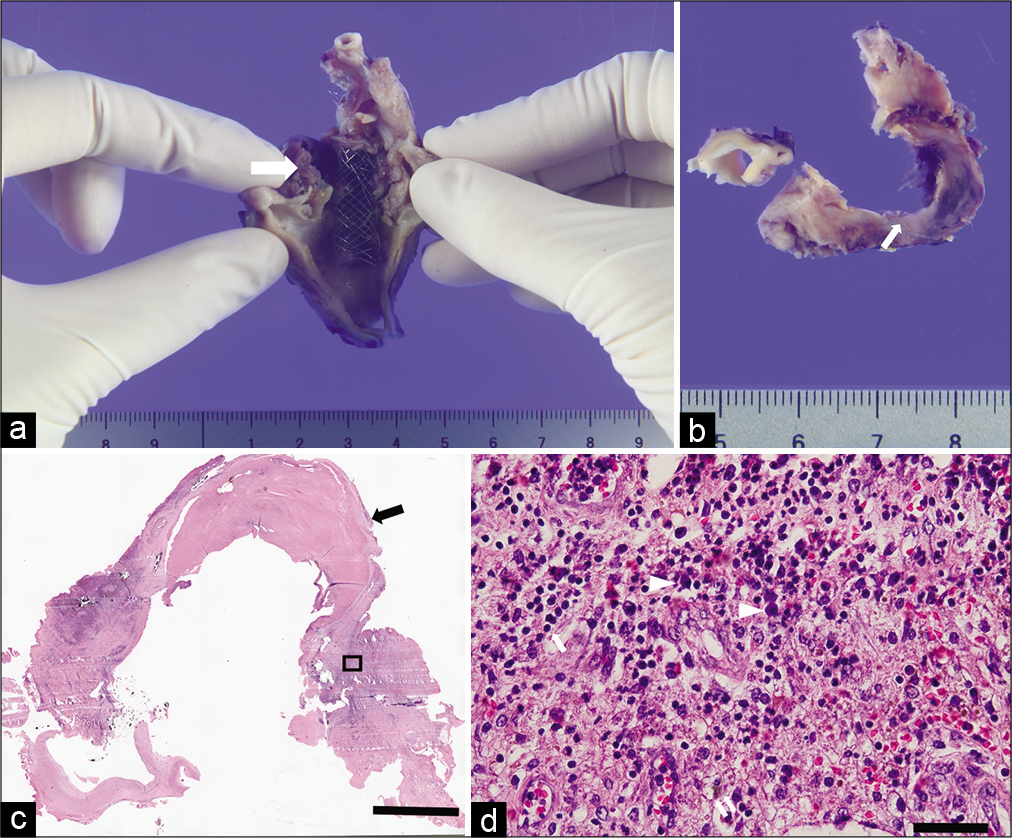
Figure 4:
Histopathological images. (a) Overview image of the excised pseudoaneurysm and stent. Arrow shows the cutting level of the axial image. (b) Axial image cut at the level of the arrow in (a) showing rupture of intima (arrow). (c) Hematoxylin-eosin staining. The arrow shows the vessel wall consisting of a clot. The black bar equals 5 mm. (d) Magnified image of the square region in (c). The arrow shows a neutrophil. Arrowheads show plasma cells. Double arrow shows a hemosiderin-laden macrophage. The black bar equals 50 m.
DISCUSSION
We report a case of stent infection with pseudoaneurysm formation 3 months after CAS that was treated by replacing the infected stent and pseudoaneurysm with a PTFE synthetic vessel graft.
Stent infection after CAS is an extremely rare complication. Lejay et al. searched for studies evaluating infection in supra-aortic trunks published between 1997 and 2017 and found only eight cases of stent infection in the carotid artery.[
In the previous reports, authors discussed some potential causes of carotid stent infections.[
Although staphylococci are the most frequently encountered microorganism in cases of stent infections involving supraaortic trunks, comprising about 60% of cases,[
The mortality rates when using conservative antibiotic therapy to treat stent infections is high overall (50%) and for non-coronary stents is 14.3%,[
CONCLUSION
Although the risk of reinfection is high, stent/ pseudoaneurysm resection and in situ reconstruction with a PTFE synthetic vessel graft might be one of the best options for patients suffering stent infection after CAS, particularly in older individuals in poor condition.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Grant-in-Aid for Young Scientist (No. 18K16582 to T.O.) from the Japan Society for the Promotion of Science (JSPS).
Conflicts of interest
There are no conflict of Interest.
Acknowledgments
This work was supported by a Grant-in-Aid for Early-Career Scientists from the Japan Society for the Promotion of Science to TO (18K16582).
We thank Dr. Hiroshi Nishimura from department of Otorhinolaryngology-Head and Neck Surgery, National Hospital Organization Osaka National Hospital and Dr. Takumi Arika from department of Oral and Maxillofacial Surgery, National Hospital Organization Osaka National Hospital for helping our surgery.
We thank Leonie McKinlay, DVM, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
References
1. Bandyk DF, Novotney ML, Johnson BL, Back MR, Roth SR. Use of rifampin-soaked gelatin-sealed polyester grafts for in situ treatment of primary aortic and vascular prosthetic infections. J Surg Res. 2001. 95: 44-9
2. Bosman WM, van der Burg BL, Schuttevaer HM, Thoma S, Joosten PP. Infections of intravascular bare metal stents: A case report and review of literature. Eur J Vasc Endovasc Surg. 2014. 47: 87-99
3. Curtis JJ, Stoney WS, Alford WC, Burrus GR, Thomas CS. Intimal hyperplasia. A cause of radial artery aortocoronary bypass graft failure. Ann Thorac Surg. 1975. 20: 628-35
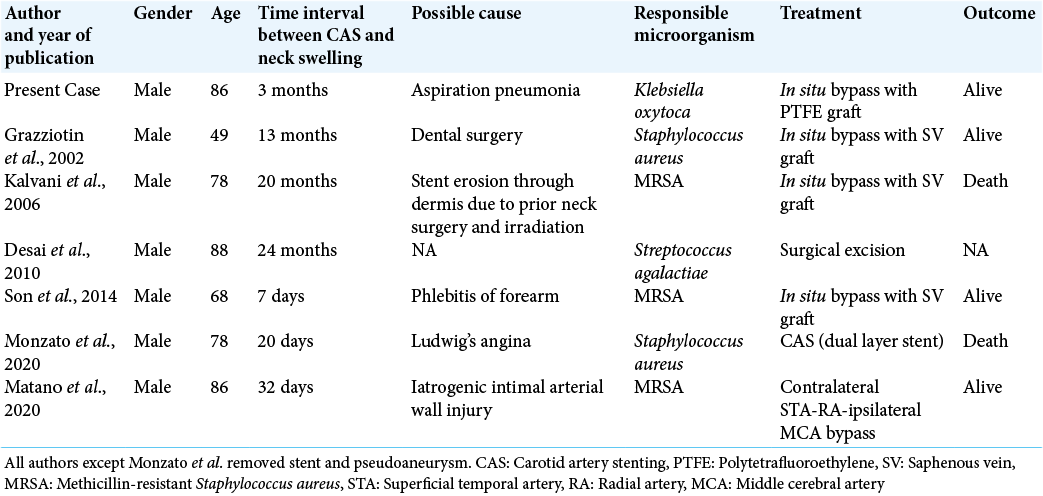
4. Desai JA, Husain SF, Islam O, Jin AY. Carotid artery stent infection with Streptococcus agalactiae. Neurology. 2010. 74: 344
5. Edwards WH, Martin RS, Jenkins JM, Edwards WH Sr, Mulherin JL. Primary graft infections. J Vasc Surg. 1987. 6: 235-9
6. Frasca D, Blomberg BB. Aging affects human B cell responses. J Clin Immunol. 2011. 31: 430-5
7. Grazziotin MU, Strother CM, Turnipseed WD. Mycotic carotid artery pseudoaneurysm following stenting-a case report and lessons learned. Vasc Endovascular Surg. 2002. 36: 397-401
8. Houkin K, Kamiyama H, Kuroda S, Ishikawa T, Takahashi A, Abe H. Long-term patency of radial artery graft bypass for reconstruction of the internal carotid artery. Technical note. J Neurosurg. 1999. 90: 786-90
9. Illuminati G, Pizzardi G, Pasqua R, Nardi P, Calio FG, Ricco JB. Long-term results of polytetrafluoroethylene versus saphenous vein repair of degenerative carotid artery aneurysm. J Vasc Surg. 2020. 72: 1413-20
10. Ishishita Y, Tanikawa R, Noda K, Kubota H, Izumi N, Katsuno M. Universal extracranial-intracranial graft bypass for large or giant internal carotid aneurysms: Techniques and results in 38 consecutive patients. World Neurosurg. 2014. 82: 130-9
11. Kaviani A, Ouriel K, Kashyap VS. Infected carotid pseudoaneurysm and carotid-cutaneous fistula as a late complication of carotid artery stenting. J Vasc Surg. 2006. 43: 379-82
12. Kilic A, Arnaoutakis DJ, Reifsnyder T, Black JH, Abularrage CJ, Perler BA. Management of infected vascular grafts. Vasc Med. 2016. 21: 53-60
13. Kiriyama K, Kamada K. Carotid artery reconstruction using a synthetic graft following repair of an infected carotid aneurysm in an elderly patient: A case report. Surg Cereb Stroke. 2017. 45: 398-402
14. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016. 16: 626-38
15. Kocaeli H, Andaluz N, Choutka O, Zuccarello M. Use of radial artery grafts in extracranial-intracranial revascularization procedures. Neurosurg Focus. 2008. 24: E5
16. Lejay A, Koncar I, Diener H, de Ceniga MV, Chakfe N. Postoperative infection of prosthetic materials or stents involving the supra-aortic trunks: A comprehensive review. Eur J Vasc Endovasc Surg. 2018. 56: 885-900
17. LeMaire SA, Coselli JS. Options for managing infected ascending aortic grafts. J Thorac Cardiovasc Surg. 2007. 134: 839-43
18. Liu J, Zeng Q, Huang JJ, Hu GH. Management of infected carotid artery rupture. Eur Arch Otorhinolaryngol. 2014. 271: 1723-8
19. Manzato LB, Cordeiro R, Karam O, Figini VA, Klock C, Angeliero VE. Stent infection after carotid angioplasty-treatment with dual layer stent. Brain Circ. 2020. 6: 215-8
20. Marcus HJ, Patel HC, Kirkpatrick PJ. Graft aneurysm complicating an extracranial-intracranial bypass. Br J Neurosurg. 2009. 23: 548-50
21. Matano F, Suzuki M, Mizunari T, Yamada T, Murai Y, Morita A. Radial artery graft for giant common carotid artery pseudoaneurysm after carotid artery stenting. World Neurosurg. 2020. 139: 401-4
22. Matsukawa H, Tanikawa R, Kamiyama H, Tsuboi T, Noda K, Ota N. Risk factors for neurological worsening and symptomatic watershed infarction in internal carotid artery aneurysm treated by extracranial-intracranial bypass using radial artery graft. J Neurosurg. 2016. 125: 239-46
23. Nishimura K, Nakamura Y, Harada S, Saiki M, Marumoto A, Kanaoka Y. Saphenous vein graft aneurysm after coronary artery bypass grafting. Ann Thorac Cardiovasc Surg. 2009. 15: 61-3
24. Pasic M, Schwitter J, Vogt M, Carrel T, von Segesser L, Turina M. Ruptured mycotic extracranial carotid aneurysm treated by excision, PTFE graft interposition, and local antibiotic application-a case report. Vasc Surg. 1992. 26: 421-5
25. Quinones-Hinojosa A, Du R, Lawton MT. Revascularization with saphenous vein bypasses for complex intracranial aneurysms. Skull Base. 2005. 15: 119-32
26. Ramdon A, Martinez-Singh K, Hnath JC, Chang BB, Darling RC. Long-term patency of venous and prosthetic conduits for ipsilateral internal carotid artery bypass. J Vasc Surg. 2019. 70: 1935-41
27. Schmitt DD, Bandyk DF, Pequet AJ, Towne JB. Bacterial adherence to vascular prostheses. A determinant of graft infectivity. J Vasc Surg. 1986. 3: 732-40
28. Sekhar LN, Bucur SD, Bank WO, Wright DC. Venous and arterial bypass grafts for difficult tumors, aneurysms, and occlusive vascular lesions: Evolution of surgical treatment and improved graft results. Neurosurgery. 1999. 44: 1207-23
29. Son S, Choi NC, Choi DS, Cho OH. Carotid stent infection: A rare but potentially fatal complication of carotid artery stenting. BMJ Case Rep. 2014. 2014: bcr2014011143
30. Thibodeaux LC, James KV, Lohr JM, Welling RE, Roberts WH. Infection of endovascular stents in a swine model. Am J Surg. 1996. 172: 151-4
31. Weng NP. Aging of the immune system: How much can the adaptive immune system adapt?. Immunity. 2006. 24: 495-9
32. Wilson WR, Bower TC, Creager MA, Amin-Hanjani S, O’Gara PT, Lockhart PB. Vascular graft infections, mycotic aneurysms, and endovascular infections: A scientific statement from the American Heart Association. Circulation. 2016. 134: e412-60
33. Yasim A, Gul M, Ciralik H, Ergun Y. Gelatin-sealed dacron graft is not more susceptible to MRSA infection than PTFE graft. Eur J Vasc Endovasc Surg. 2006. 32: 425-30