- Department of Neurosurgery, Yokohama Rosai Hospital, Yokohama, Japan.
Correspondence Address:
Takashi Shuto, Department of Neurosurgery, Yokohama Rosai Hospital, Yokohama, Japan.
DOI:10.25259/SNI_815_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takashi Shuto, Shigeo Matsunaga, Jo Sasame. Stereotactic intensity-modulated radiotherapy for skull base meningioma using the HybridArc with Novalis STx system. 08-Dec-2023;14:420
How to cite this URL: Takashi Shuto, Shigeo Matsunaga, Jo Sasame. Stereotactic intensity-modulated radiotherapy for skull base meningioma using the HybridArc with Novalis STx system. 08-Dec-2023;14:420. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12660
Abstract
Background: Skull base meningiomas are often difficult to remove completely with preserved nerve function and may require radiation therapy. However, the Gamma Knife is unsuitable for large tumor volume or the optic nerve, which is difficult to identify on imaging. We report the results of stereotactic radiotherapy with HybridArc using Novalis STx for skull base meningiomas.
Methods: We retrospectively examined 28 patients with skull base meningioma who underwent stereotactic radiotherapy (54 Gy/30 fractions) with HybridArc.
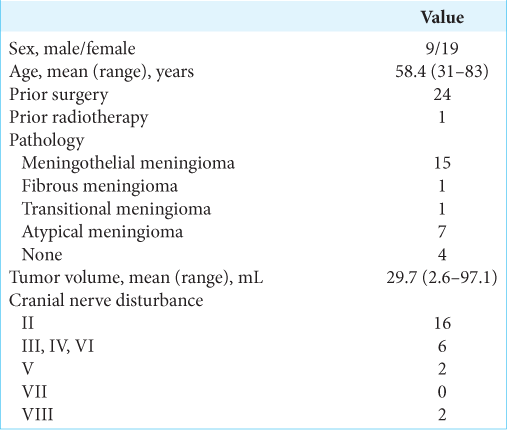
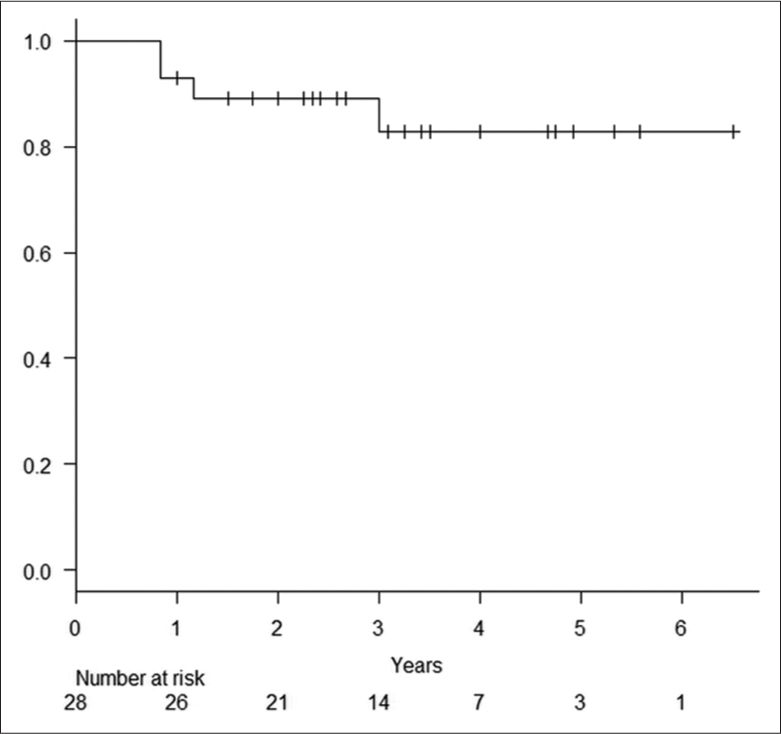
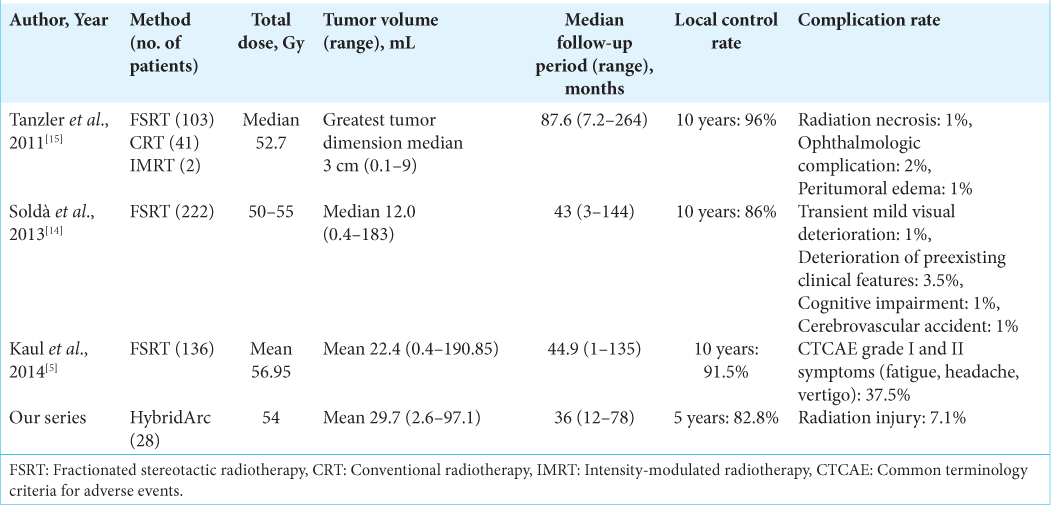
Results: The 28 patients, nine males and 19 females, were aged 31–83 years (mean 58.4 years), and the tumor volume was 2.6–97.1 mL (mean 29.7 mL). HybridArc irradiation was performed with D95 54 Gy/30 fractions for all patients with a median follow-up period of 36.0 months (range: 12–78 months). Tumor control rates at 1, 2, and 5 years after radiotherapy were 92.6%, 89.1%, and 82.8%, respectively. Only one non-atypical meningioma remained uncontrolled; thus, the tumor control rate for non-atypical meningioma at 1, 2, and 5 years was 94.1%. Tumor control rates for atypical meningioma at 1, 2, and 5 years were 85.7%, 71.4%, and 53.6%, respectively, significantly worse than for non-atypical meningiomas (P = 0.0395). Radiation injury was observed in two cases (7.1%). Visual field defects were observed in 16 patients, and diplopia in 6. Visual field and diplopia improvements were achieved in 5 and 2 patients, respectively (with overlap).
Conclusion: Stereotactic radiotherapy (54 Gy/30 fractions) with HybridArc using Novalis STx is a safe and effective approach for relatively large skull base meningiomas.
Keywords: HybridArc, Intensity-modulated radiotherapy, Novalis STx, Skull base meningioma, Stereotactic radiotherapy
INTRODUCTION
A complete surgical removal of skull base meningiomas with preservation of neurological function is often difficult; radiotherapy may be used in combination or as an alternative to surgery. However, stereotactic radiosurgery (SRS), such as Gamma Knife surgery, is not suitable for large tumors that cause severe compression of the brain stem or optic apparatus due to the high risk of exceeding the tolerable dose of radiation for nearby structures, especially the optic nerve.
Stereotactic multi-fraction radiotherapy using the Novalis STx system is suitable for relatively large lesions that compress the optic nerve/chiasm and brain stem. This system is a high-performance linear accelerator (Linac), which enables highly accurate irradiation with the Brainlab ExacTrac system, a positioning device that combines X-ray and infrared radiation, and a robotic couch with 6-axis correction. The Novalis STx system enables high-precision SRS incorporating various methods, including normal rotational body irradiation (dynamic conformal arc), intensity-modulated radiotherapy (IMRT) using beams, and Brainlab HybridArc, a method of irradiation combining several dynamic arcs and IMRT beams.[
MATERIALS AND METHODS
We retrospectively investigated 28 patients who underwent stereotactic multi-fraction radiotherapy (54 Gy/30 fractions) using the Novalis STx system and were followed up for 12 months or longer. Patients with treatment failure within 12 months were also included, but those with anaplastic meningioma were excluded from the study. The selection criteria for this treatment were as follows:
Skull base meningioma that was difficult to remove by surgery completely or surgery refused by the patient Cases where the tumor volume is so large that SRS or hypofractionated radiotherapy is considered to carry a high risk of radiation injury Cases where it is difficult to identify the optic tract (optic nerve/optic chiasm) on magnetic resonance imaging due to tumor size and/or severe brainstem compression.
Thin-slice imaging was performed; mainly, contrast-enhanced T1-weighted images, T2-weighted images, and fluid-attenuated inversion recovery (FLAIR) images were acquired, and patient-specific plastic shells were constructed. Treatment plans were generated using Brainlab iPlan® software. The planning target volume (PTV) varied from case to case, but – in many cases – the PTV was defined as gross tumor volume with a 1–2 mm margin added, and HybridArc irradiation was performed by combining a dynamic conformal arc with two IMRT beams in each arc.
Posttreatment tumor size was evaluated with contrast-enhanced T1-weighted imaging performed every six months, and tumor volume was measured using iPlan®. Occurrence and deterioration of cerebral edema around the tumor were monitored using T2-weighted or FLAIR imaging. Toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, produced by the U.S. National Cancer Institute, Cancer Therapy Evaluation Program (
A partial response was defined as a ≥10% decrease in tumor volume, and a stable or partial response was considered to indicate tumor control. Progression was defined as tumor growth of ≥10%. Tumor progression and requirement of salvage therapy due to radiation injury were defined as uncontrolled.
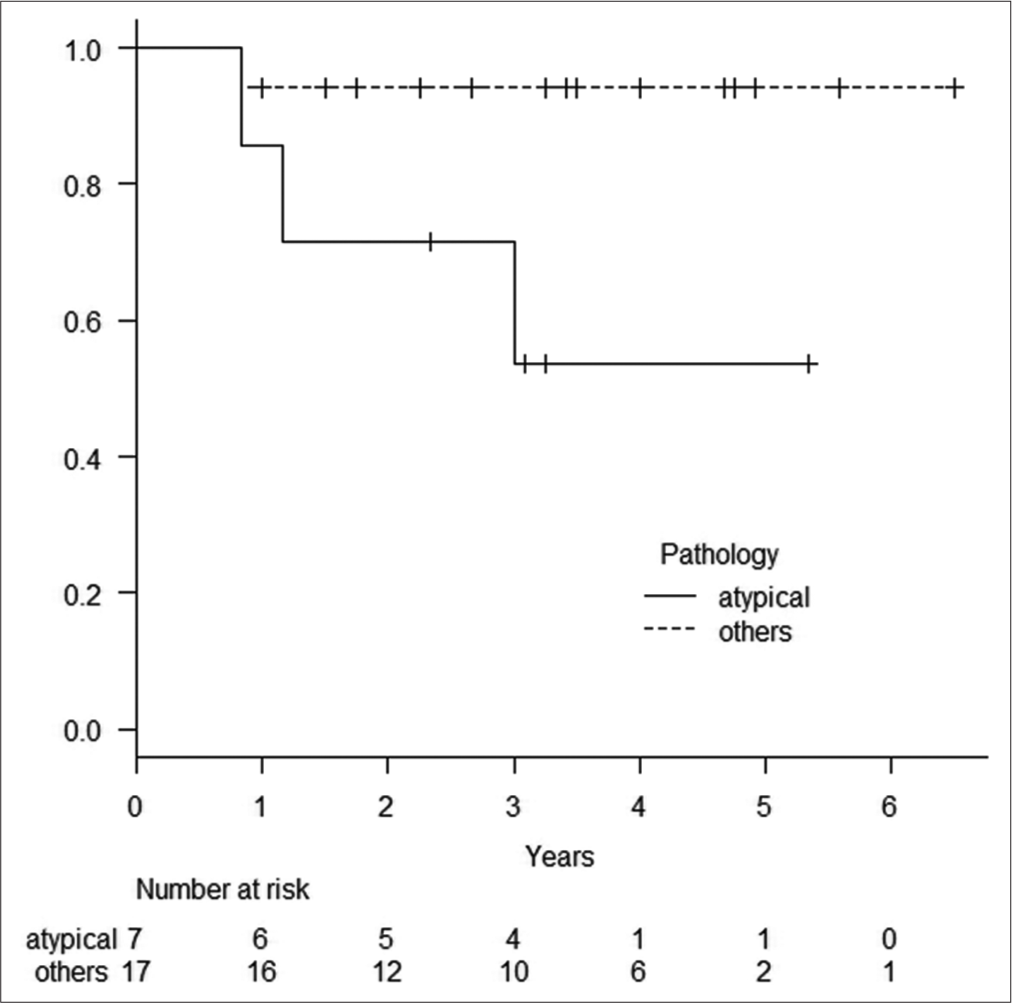
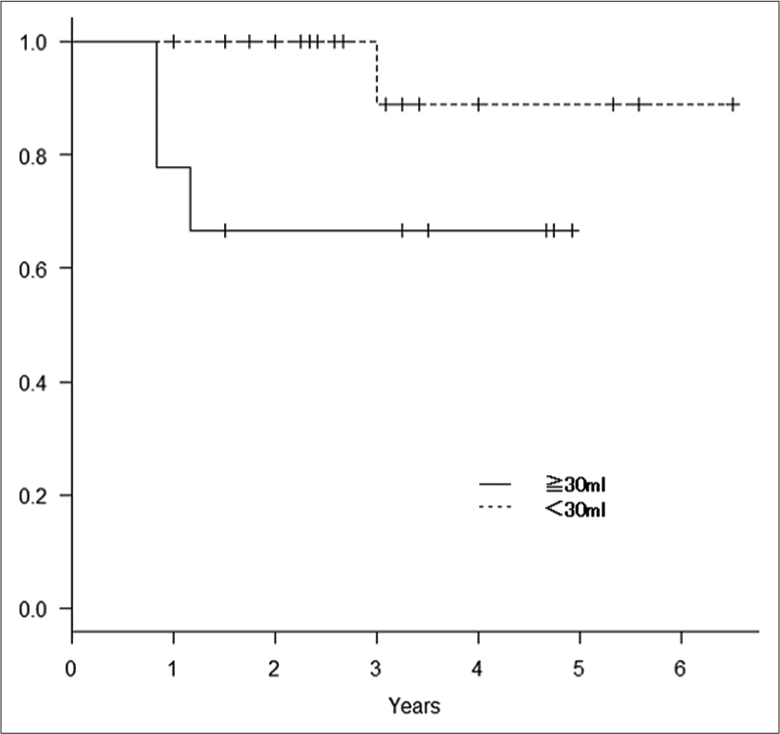
Tissue type (atypical or not), age (≥50 years or < 50 years), sex, and tumor volume (≥30 mL or <30 mL) were analyzed as prognostic factors related to tumor control. The Cox proportional hazards model was used to identify factors affecting survival time. All statistical analyses were performed with EZR (Easy R; Saitama Medical Center, Saitama, Japan; and Jichi Medical University, Shimotsuke, Tochigi, Japan).[
RESULTS
Twenty-four cases (all except the four cases without histological diagnoses) were assessed for atypical or non-atypical meningioma [
Four cases were uncontrolled in this study. Two cases (one case was an atypical meningioma) developed deteriorating cerebral edema around the tumor ten months after radiotherapy. However, this condition was present in both cases before treatment, so one patient underwent direct surgery while the other was transferred to the referring physician. The other two uncontrolled cases were categorized as such because salvage radiotherapy was required: One due to intracranial disseminated metastasis and the other due to tumor growth. Both of these cases were atypical meningiomas.
Compression of the optic nerve/chiasm or invasion of the cavernous sinus by the tumor was observed in 27 of the 28 cases. Visual field defects or visual impairment were observed in 16 patients, and diplopia was observed in six patients [
Radiation injury, identified by the presence of hyperintensity on T2-weighted imaging, was observed in two patients (7.1%, including the uncontrolled cases). Cerebral edema around the tumor was already present in both patients before radiotherapy, and deterioration occurred in both cases 10 months later.
Imaging follow-up at ≥36 months postoperatively was carried out for 15 patients. For these patients, the tumor volume before irradiation was 21.1 ± 15.2 mL, and the tumor volume at the final follow-up (rage: 36–78 months, mean: 49.2 months) was 15.3 mL ± 13.6 mL. A significant reduction in tumor volume was obtained in these 15 patients (P = 0.01, paired t-test).
DISCUSSION
Radiation tolerance of the optic nerve/optic chiasm
The most clinically significant issue in radiotherapy for parasellar-sphenoid ridge meningioma is avoidance of radiation damage to the optic nerve and optic chiasm. SRS studies show that these structures can tolerate around 8–12 Gy.[
The α/β values of the brain and optic nerve are thought to be as low as ~2 Gy, so each fraction must be <2 Gy to preserve visual function when treating large parasellar meningiomas. An analysis of 34 papers concluded that delivery of 10 Gy in a single session, 20 Gy in three fractions, or 25 Gy in five fractions resulted in a 1% risk of radiation-induced optic nerve/chiasm neuropathy (RION).[
Stereotactic hypofractionated radiotherapy for meningioma
Stereotactic irradiation provides a steep dose gradient. Thus, if the tumor volume is not large and the distance between the tumor and optic nerve/optic chiasm is 2–3 mm or more, SRS with a marginal dose of 13–16 Gy is possible. However, if the tumor is in contact with or compressing the optic nerve/optic chiasm, the dose to the optic nerve on the tumor surface is almost equal to the marginal dose. Therefore, the dose in each fraction must be reduced to avoid RION. The optimal dose for meningiomas is about 25–30 Gy delivered in five fractions, and good tumor control can be expected.[
Stereotactic multi-fraction radiotherapy with the Novalis STx system
Tumor control of large skull base meningiomas that are difficult to remove completely surgically can often be obtained using stereotactic multi-fraction radiotherapy with fractions of 1.8–2 Gy and a total dose of 50–54 Gy [
Improvements in visual function can be expected after irradiation.[
Imaging follow-up data were available for a significant proportion of the study population, and the tumor volumes observed at the final follow-up indicated a gradual and long-term reduction in size.
In our series, four out of five uncontrolled cases were atypical meningiomas. However, SRS using HybridArc (54 Gy/30 fractions) proved highly effective for grade 1 meningiomas, suggesting a high likelihood of tumor control. Our findings indicate that the HybridArc technique is particularly effective for skull base meningiomas.
One drawback of this approach is the extended treatment timeline, which exceeds six weeks due to treatment plan preparation and verification. Radiation-induced injuries, manifested as T2-weighted hyperintensity, occurred in only two cases within our series. These injuries were observed in relatively large tumors that already exhibited cerebral edema before irradiation, necessitating careful management.
Conversely, grade 1 meningiomas without preexisting cerebral edema carried a significantly lower risk of radiation injury, even in the case of large tumors. Therefore, stereotactic multi-fraction radiotherapy utilizing HybridArc with Novalis STx is considered a safe treatment method for these tumors.
CONCLUSION
Stereotactic multi-fraction radiotherapy (54 Gy/30 fractions) with HybridArc using the Novalis STx system minimizes the risk of radiation damage, leading to visual dysfunction when treating relatively large skull base meningiomas that compress the optic nerve and optic chiasm. A high tumor control rate can be expected with the application of this system.
Ethical approval
Not applicable.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We are deeply grateful to all those who helped with this study, especially those who provided data.
References
1. Eckert F, Clasen K, Kelbsch C, Tonagel F, Bender B, Tabatabai G. Retrospective analysis of fractionated intensity-modulated radiotherapy (IMRT) in the interdisciplinary management of primary optic nerve sheath meningiomas. Radiat Oncol. 2019. 14: 240
2. Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991. 21: 109-22
3. Han J, Girvigian MR, Chen JC, Miller MJ, Lodin K, Rahimian J. A comparative study of stereotactic radiosurgery, hypofractionated, and fractionated stereotactic radiotherapy in the treatment of skull base meningioma. Am J Clin Oncol. 2014. 37: 255-60
4. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013. 48: 452-8
5. Kaul D, Budach V, Misch M, Wiener E, Exner S, Badakhshi H. Meningioma of the skull base: Long-term outcome after image-guided stereotactic radiotherapy. Cancer Radiother. 2014. 18: 730-5
6. Maclean J, Fersht N, Bremner F, Stacey C, Sivabalasingham S, Short S. Meningioma causing visual impairment: Outcomes and toxicity after intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2013. 85: e179-86
7. Milano MT, Grimm J, Soltys SG, Yorke E, Moiseenko V, Tomé WA. Single-and multi-fraction stereotactic radiosurgery dose tolerances of the optic pathways. Int J Radiat Oncol Biol Phys. 2021. 110: 87-99
8. National Cancer Institute. Common terminology criteria for adverse events (CTCAE) v5.0. Available from: https://ctep.cancer.gov/Protocoldevelopment/electronic_applications/ctc.htm#ctc_50 [Last accessed on 2022 Mar 17].
9. Navarria P, Pessina F, Cozzi L, Clerici E, Villa E, Ascolese AM. Hypofractionated stereotactic radiation therapy in skull base meningiomas. J Neurooncol. 2015. 124: 283-9
10. Patibandla MR, Lee CC, Tata A, Addagada GC, Sheehan JP. Stereotactic radiosurgery for WHO grade I posterior fossa meningiomas: Long-term outcomes with volumetric evaluation. J Neurosurg. 2018. 129: 1249-59
11. Pollock BE, Link MJ, Leavitt JA, Stafford SL. Dose-volume analysis of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Neurosurgery. 2014. 75: 456-60
12. Robar JL, Thomas C. HybridArc: A novel radiation therapy technique combining optimized dynamic arcs and intensity modulation. Med Dosim. 2012. 37: 358-68
13. Shrieve DC, Hazard L, Boucher K, Jensen RL. Dose fractionation in stereotactic radiotherapy for parasellar meningiomas: Radiobiological considerations of efficacy and optic nerve tolerance. J Neurosurg. 2004. 101: 390-5
14. Soldà F, Wharram B, De Ieso PB, Bonner J, Ashley S, Brada M. Long-term efficacy of fractionated radiotherapy for benign meningiomas. Radiother Oncol. 2013. 109: 330-4
15. Tanzler E, Morris CG, Kirwan JM, Amdur RJ, Mendenhall WM. Outcomes of WHO Grade I meningiomas receiving definitive or postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2011. 79: 508-13