- Division of Neurosurgery, Beth Israel Deaconess Medical Center, Harvard Medical School, 110 Francis Street, Boston, MA 02215, USA
- Department of Radiation Oncology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave., Boston, MA 02215, USA
- Koch Institute for Integrative Cancer Biology, Massachusetts Institute of Technology, 500 Main Street, Cambridge, MA 02139, USA
- Division of Neurosurgery, University of California Sand Diego, 200 West Arbor Drive, San Diego, CA 92103, USA
- Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave., Boston, MA 02215, USA
Correspondence Address:
Ekkehard M. Kasper
Division of Neurosurgery, Beth Israel Deaconess Medical Center, Harvard Medical School, 110 Francis Street, Boston, MA 02215, USA
DOI:10.4103/2152-7806.163315
Copyright: © 2015 Christ SM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Christ SM, Mahadevan A, Floyd SR, Lam FC, Chen CC, Wong ET, Kasper EM. Stereotactic radiosurgery for brain metastases from malignant melanoma. Surg Neurol Int 20-Aug-2015;6:
How to cite this URL: Christ SM, Mahadevan A, Floyd SR, Lam FC, Chen CC, Wong ET, Kasper EM. Stereotactic radiosurgery for brain metastases from malignant melanoma. Surg Neurol Int 20-Aug-2015;6:. Available from: http://surgicalneurologyint.com/surgicalint_articles/stereotactic-radiosurgery-for-brain-metastases-from-malignant/
Abstract
Background:Surgical resection and stereotactic radiosurgery (SRS) are well-established treatment methods for patients with brain metastases, yet their respective roles in the management of brain metastases remain incompletely defined.
Methods:To report on the role of SRS in the treatment of patients with brain metastases from malignant melanoma, a retrospective analysis of 381 intracranial melanoma metastases in 103 consecutive patients who underwent SRS between 2005 and 2011 at Beth Israel Deaconess Medical Center was conducted. The Cyberknife® SRS system was used to treat all patients. Clinical, technical, and radiographic data were recorded at presentation and on follow-up.
Results:The patient cohort consisted of 40 female (39%) and 63 male (61%) patients with a median age of 57 years. The median overall survival from the time of radiosurgery for the entire patient cohort was 7.6 months. The local control rate at 1-year was 72% for the patients who received surgery followed by SRS and 55% for the entire patient population. Surgery followed by SRS was associated with significantly improved overall survival compared with SRS alone or whole-brain radiation therapy followed by salvage SRS (P
Conclusions:Both surgery plus SRS and SRS provide comparable local control. Despite the difference in lesion size in the subgroups who received surgery plus SRS and radiosurgery alone, similar outcomes were achieved in both groups, suggesting that surgical treatment of larger lesions can yield results that are not significantly different from small lesions treated by SRS alone.
Keywords: Brain metastasis, CyberKnife, melanoma, stereotactic radiosurgery
INTRODUCTION
Cutaneous malignant melanoma (MM) is the fifth most common cancer in males and the sixth most common form of cancer in females.[
MATERIALS AND METHODS
Study design and data collection
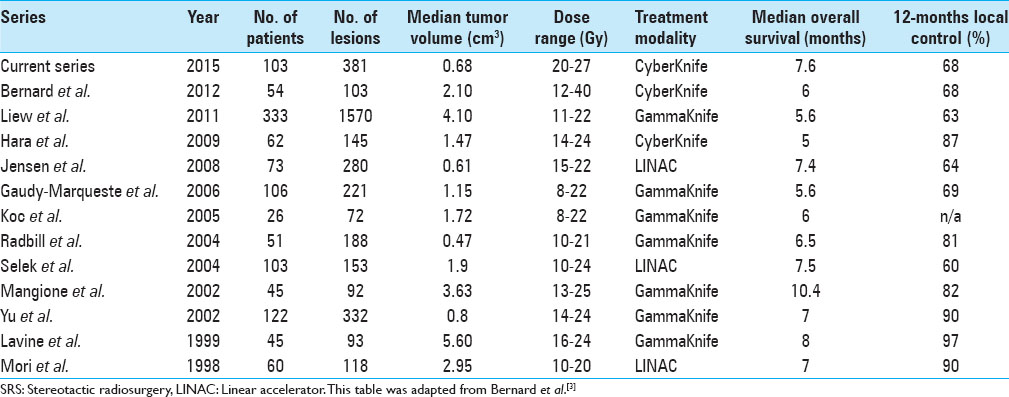
In planning and designing this study, similar series served as inspirational source and we closely looked at the studies of Bernard et al., Liew et al., Hara et al., Gaudy-Marqueste et al., Selek et al., and others.[
Patient population
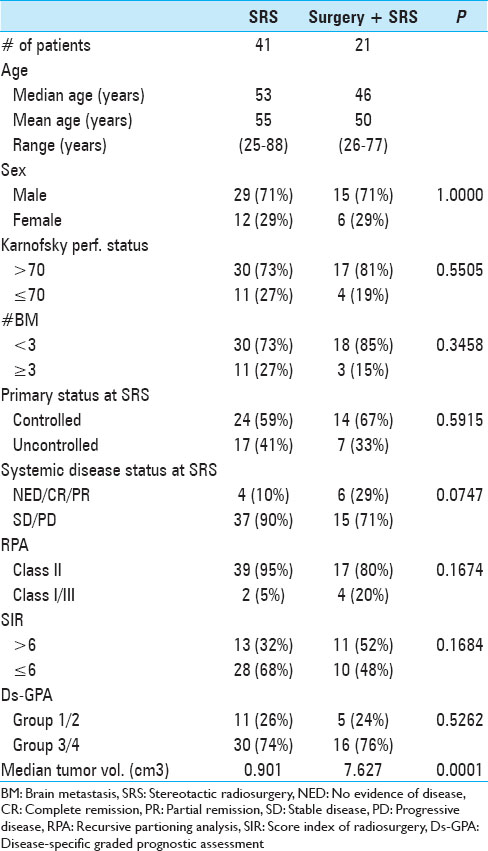
The study cohort is comprised of a total of 381 distinct lesions from 103 patients who were treated with CK SRS (Accuray, Sunnyvale, CA) by the Departments of Radiation Oncology and Neurosurgery at BIDMC between January 2005 and December 2011. Of those, 41 patients (40%) received SRS alone, 21 patients (19%) were treated with SRS as salvage therapy after WBRT and 21 (19%) patients underwent postoperative adjuvant SRS treatment to the resection cavity after conventional image-guided open surgical resection. Prior to treatment with SRS, all patients in the cohort underwent a complete primary as well as systemic work-up. In 94% of the cases, cerebral MM metastasis was diagnosed by magnetic resonance imaging (MRI). For six patients (6%), computed tomography (CT) with and without i.v. contrast was used, as MRI imaging was contraindicated, for example, in cases of contrast allergy (3) or in the setting of cardiac pacemaker placement (3).
Eligibility and inclusion criteria
Patients who underwent SRS met the following widely-used eligibility criteria: (a) All patients were adults; (b) all patients had histologic confirmation of MM either at the primary site or at a site of metastatic disease; (c) greatest tumor diameter was limited to tumors measuring less than 3 cm prior to resection; and (d) no major, sustained neurologic deficit due to mass effect was present during treatment time. In patients where large tumor masses were causing significant neurologic symptoms (which did not improve after corticosteroid application) a craniotomy was undertaken whenever the tumor was accessible and located in a noneloquent region of the brain.
Follow-up
First posttreatment MRI and clinical follow-up examination were routinely obtained at 1 month after SRS. Subsequent MRI scans and clinical follow-up examinations were obtained every 2–3 months. Follow-up assessment was scheduled for surveillance at set intervals even in patients who remained clinically asymptomatic. A lesion was classified as local failure in all patients where at least one of the following conditions was met: (a) An increase in lesion size in gadolinium enhanced MRI, and (b) SRS-related complications such as symptomatic hemorrhage or (c) features unclear of radiation necrosis in follow-up imaging requiring surgical intervention. Cerebral metastasis in a location other than the previous tumor locations was categorized as distant failure. Eight patients (8%) transferred care to other facilities and no radiographic follow-up data was available. These patients were excluded from the tumor control analysis. For the remaining 95 of the 103 original study patients, the clinical development was continuously recorded. Patients and lesions were included into the analysis after at least a year had passed since the final patient received radiosurgery treatment. Fairly similar criteria were used in the study by Selek et al.[
Statistical methods
Descriptive statistics as well as frequencies were obtained for multiple variables. The Fisher's exact test and the Kruskal–Wallis test were used to examine the homogeneity of patient groups. The distribution of the different treatment modalities was approximately uniform over the examined time span. Kaplan–Meier analyses were therefore performed to calculate OS, LTC, and PFS. Posttreatment time intervals were assessed in months and are based on the start date of SRS treatment and the last imaging date or the last follow-up date. For categorical variables, univariate analysis in form of the log-rank was employed. Cox proportional hazards model was used for univariate analysis of continuous variables and in the assessment of the prognostic value of different variables in multivariate analysis. Due to the unfavorable observations to variables ratio, we opted for stepwise regression by forward selection in multivariate Cox analysis with stringent criteria to avoid overfitting the data. Statistical significance was defined as a P value of less than 0.05 in both univariate and multivariate analyses. All statistical calculations were performed using the STATA 11.0 software package (STATA Corp., College Station, TX, USA).
RESULTS
Patient demographics
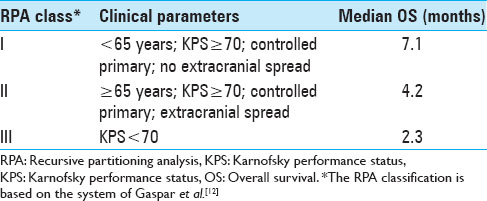
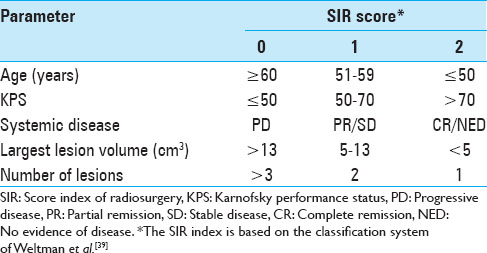
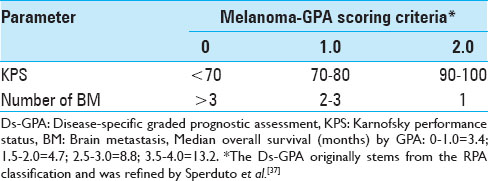
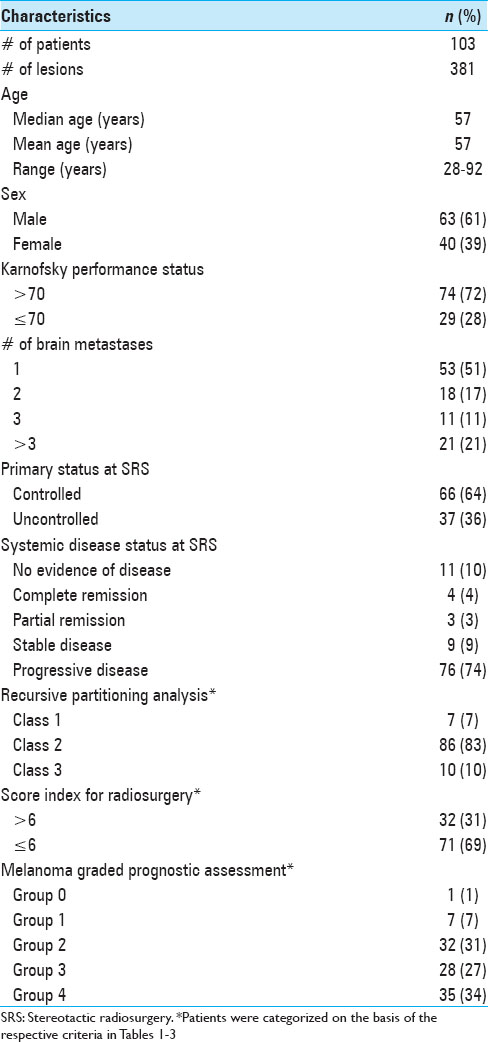
The entire patient cohort consisted of 40 female (39%) and 63 male (61%) patients, aged between 28 and 92 years (mean age 57 years, median age 57 years) at the time of their initial BM diagnosis. When the patients first presented, their Karnofsky performance status (KPS) was 90 (range, 40-100). Fifty-two patients (50.5%) presented with a single metastasis, 51 patients (49.5%) had multiple metastases at initial presentation. Twenty-one patients (20%) had four or more metastases. Three hundred and fifty (92%) lesions of this data set were located supratentorially. When SRS treatment was initially undertaken, a systemic survey was conducted in all patients, which included both a CT torso and in some cases a positron emission tomography (PET)-CT. The status of the extra-cranial melanoma was classified as controlled in 66 patients (64%). The primary skin lesions were located on the trunk in 31 patients (31%); in 29 patients (28%) the initial skin lesion was found on the extremities; 23 patients (22%) presented with lesions in the head and neck region; 2 patients (2%) had ocular melanoma, 2 patients (2%) had vaginal melanoma; and in 16 patients (15%) the primary tumor location remained unknown. At the time of initial SRS treatment, 15 patients (15%) were found to have either no evidence of systemic disease (NED) or were in complete clinical remission (CR). As Selek et al., we also classified our patients along the lines of the following commonly used prognostic indices.[
Treatment characteristics
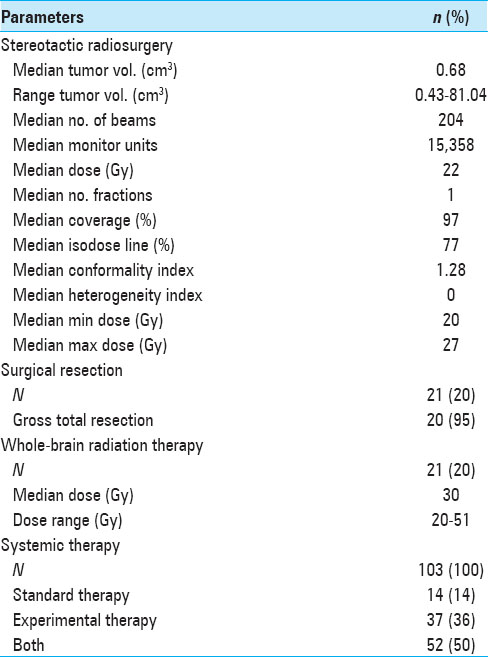
CK SRS was employed separately from any ongoing systemic treatment regimen for extracranial metastatic disease. In patients fulfilling the outlined eligibility and inclusion criteria for SRS treatment, all newly diagnosed and visible brain lesions were treated. A total of 183 SRS sessions were performed on 381 lesions in multiple plans. An average of two lesions was irradiated during each session (range, 1–7). The median individual tumor target volume was 0.68 cm3 (mean, 2.97 cm3; range, 0.04–81.04 cm3), the median prescription dose was 22 Gy (mean, 20 Gy; range, 5–22 Gy), and the median conformality index was 1.28 (mean, 1.43; range, 1.00–10.02). The median prescribed isodose line was 77% (range, 61–95%). Twenty-one patients (20%) underwent surgical resection before SRS treatment, and 21 patients (20%) had received prior WBRT (median, 30; range, 20–51 Gy). All patients received prophylactic corticosteroids (dexamethasone) and antiseizure medication (levetiracetam) during and after SRS treatment. Additional therapies included standard systemic therapy (IL-2, Ipilimumab, Dacarbacine, and Vemurafenib) in 14 patients (14%), IRB-approved experimental study therapy regimens (e.g., CTLA-AB, RAF265, ILX561, or PD-1-AB) in 37 patients (36%), or a combination of both in 52 patients (50%). Seventy-seven patients (75%) had received systemic therapy prior to SRS treatment, 20 patients (19%) had systemic treatment concurrent with or after SRS, and 6 (6%) had not yet received systemic therapy at all when the SRS treatment was undertaken. A summary of treatment parameters is presented in
Overall patient survival
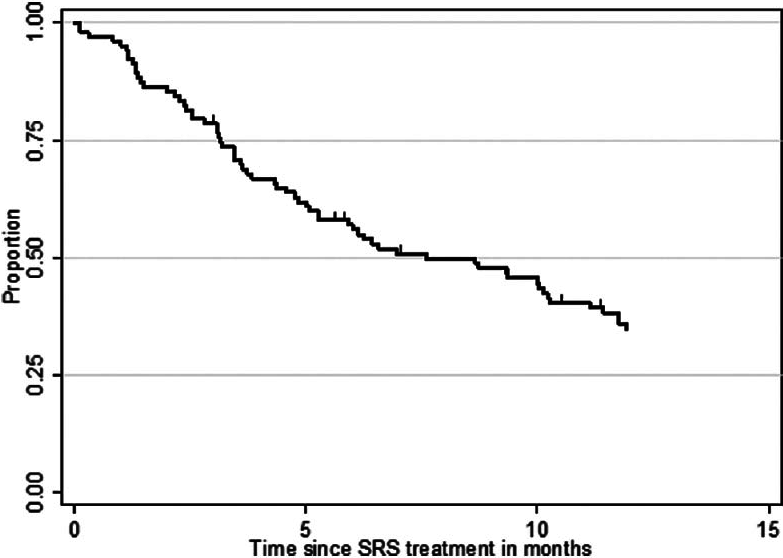
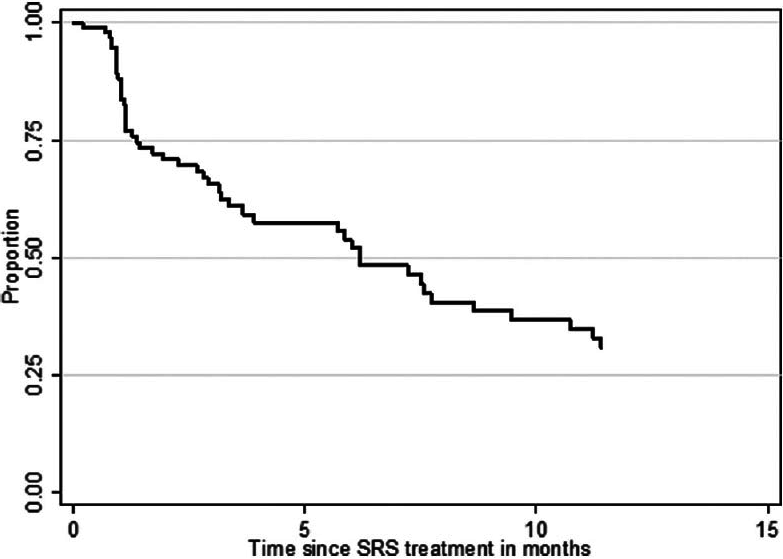
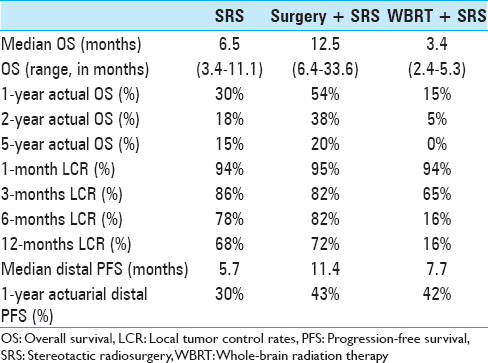
At the time of analysis (12 months after the last patient underwent SRS treatment), 86 patients (84%) were dead and 17 patients (16%) were alive. According to the data obtained in this study, 24 patients (23%) deceased from progression of brain disease (neurological death), 45 patients (44%) succumbed to systemic disease progression (nonneurological death), and in 34 patients (33%) the cause of death was unknown. The median OS after SRS was 7.6 months (95% CI 5.1–10.3 months) for the entire study population. The median OS from the diagnosis of the first BM was 11.0 months (95% CI 9.3–13.3 months) and 51.8 months (95% CI 40.8–74.8 months) from the diagnosis of the primary site malignancy. Actuarial survival rates for the entire patient cohort were 95.2% (n = 99) at 1 month, 78.6% (n = 81) at 3 months, 56.0% (n = 56) at 6 months, 34.8% (n = 32) at 12 months, and 20.2% (n = 19) at 24 months. The Kaplan–Meier plot for OS for the entire patient cohort is displayed in
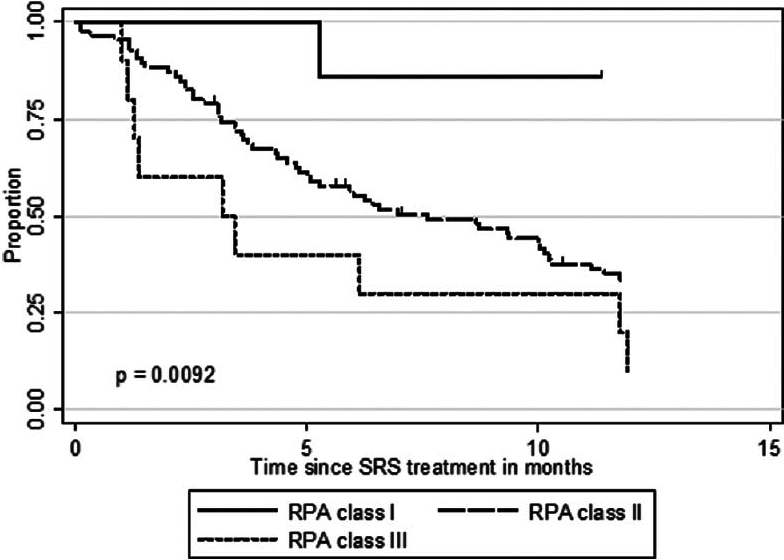
The median OS after SRS for the 52 patients with a single metastasis was 11.7 months (95% CI 8.67–15.76) compared with only 5.1 months (95% CI 3.10–6.43) for the 51 patients with multiple (n > 3) CNS metastases (P = 0.0017). Median OS was significantly different for the three RPA classes (P = 0.0092). Whereas RPA class I patients had a median OS of 33.6 months, RPA class II and III patients had a median OS of 7.6 and 3.2 months, respectively [
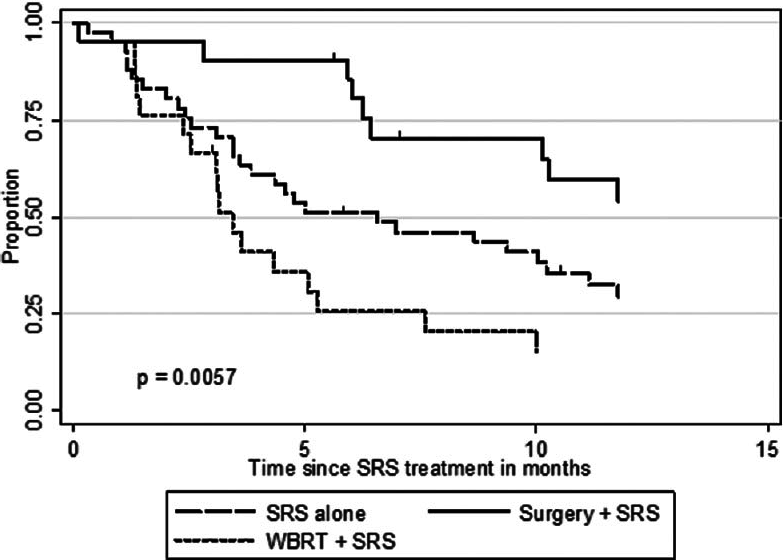
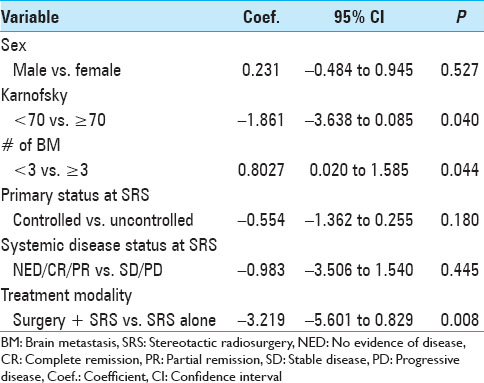
The actuarial 1-year OS rates were 29.6% for SRS alone, 54.1% for surgery plus SRS, and 15.4% for WBRT plus SRS (P = 0.0058). In univariate analysis, we found that KPS (≥70 vs. <70; P = 0.0092), the number of cerebral metastases (<3 vs. ≥3 lesions; P = 0.01), systemic disease status (PR/CR/NED vs. PD/SD; P = 0.0069), and surgical resection (P = 0.0432) were factors significantly associated with better OS. Prior WBRT (P = 0.0162) was found to be significantly associated with poor OS. The following prognostic factors were not found to be significantly associated with a survival difference in our patient cohort: Sex, age, primary status, and tumor volume. Moreover, in univariate analysis, the three prognostic indices RPA (P = 0.0092), Ds-GPA (P = 0.0012), and SIR (≥6 vs. <6; P = 0.0001) were prognostic in our patient cohort. In multivariate Cox analysis, factors associated with a significantly better OS were the number of cerebral metastases (P = 0.009), the status of systemic disease (P = 0.008), the RPA class (P = 0.006) and the Ds-GPA group (P = 0.031). The SIR score was not found to be significant in multivariate analysis. In subgroup analysis, when comparing the survival outcomes of the two patient groups who received SRS alone or surgery plus SRS, we found that the groups were fairly homogenous in terms of patient characteristics [
Analysis of patients with a single brain lesion and systemic disease control was limited by the small number of patients in these subgroups. While 22, 15, and 4 patients in the SRS, surgery plus SRS, and WBRT groups had a single brain lesion, only 4, 6, and 2 patients, respectively, had controlled systemic disease. Nevertheless, number of brain metastasis as a categorical (1 vs. multiple) or continuous variable was significant for OS in multivariable analysis independent of systemic disease status.
Local tumor control
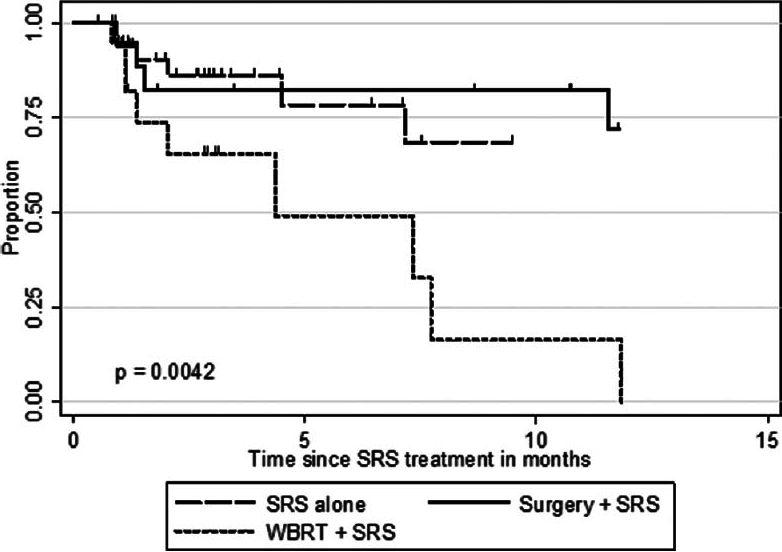
Follow-up imaging studies were available for 356 tumor lesions in 95 patients (92%). The mean patient follow-up was 10.7 months. Over the course of the entire follow-up period, local control was achieved in 71.1% of treated lesions (253 of 356 lesions). For the entire patient cohort, the 1-year incidence of local tumor control was 54.9%. Of the 41 patients who received SRS alone, local failure was noted in 14 patients (34.1%) in at least one lesion at some point in time, and 6 patients (28.6%) in the surgery group showed evidence of recurrent disease at the treatment site sometime during the follow-up period. In this series, the 1-year LCR was higher for patients who underwent SRS alone (68.5%) or surgery plus SRS (71.8%) compared to patients who received WBRT (16.4%) (P = 0.0042).
While the most significant variable for LCR in univariate log-rank analysis (P = 0.000) and multivariate Cox regression (P = 0.0053) was tumor volume, the number of BM did not significantly affect local tumor control in our patient cohort. Of all analyzed variables, tumor volume (P = 0.0210) and surgical treatment (P = 0.0219) significantly affected local PFS in multivariate analysis.
Distant progression-free survival
New brain lesions were discovered in 58 (56%) of 103 patients over the course of follow-up. After SRS treatment, a median of 6.2 months (95% CI 3.37–9.47) elapsed until distal failure occurred. Median distal PFS for patients who received SRS alone, surgery plus SRS, and WBRT plus SRS were 5.7, 11.4, and 7.7 months, respectively. Actuarial rates, capturing freedom from new brain lesions at other locations was 88% at 1 month, 66% at 3 months, 52% at 6 months, and 31% at 12 months after SRS [
In Cox analysis, new cerebral lesions occurred more likely in patients with a worse extracranial disease status (P = 0.043). Outcome characteristics of this series have been summarized in
DISCUSSION
Overall survival and prognostic factors
The primary goal of this study is to report our experience in the treatment of patients with BM from MM treated with SRS, explore the associated role of surgery and prior WBRT and to correlate our findings with previous reports in the literature. Typical therapeutic algorithms for patients with BMs nowadays comprise WBRT, SRS, surgery or a combination of these modalities.[
Surgical resection
The gold standard in patients with one accessible BM, good performance status and limited extracranial disease remains the surgical resection.[
Whole-brain radiation therapy
Traditionally, corticosteroids and WBRT were used to treat BMs.[
Local tumor control
Over the past several years, numerous studies have demonstrated that SRS is an effective treatment option for patients with BM from MM. A comprehensive review of such studies has been provided by Hanson et al., which we repeatedly consulted while compiling the following figures.[
The difficulty of defining and calculating local control rates
Cumulative research shows that LCRs of BM from radioresistant tumor entities like MM and RCC range between 50% and 100%. This huge range is neither entirely explained by varying degrees of expertise at different centers nor by the selection of different patient populations. Instead, it might at least be partially attributable to variations in definitions of local control, differing imaging follow-up schedules, and different methods of calculating LCRs. Just like in the study undertaken by Selek et al., we used stringent and conservative criteria with respect to those three aspects.[
A treatment algorithm
Patients with BM from MM are frequently classified into different treatment groups. Gaudy-Marqueste et al. have outlined four stylized clinical scenarios which treating clinicians are usually confronted with.[
Limitations of this study
Bernard et al. have pointed out that studies of this format always suffer from the common biases present in retrospective analyses and moreover mentioned several shortcomings which constitute problems for our study as well.[
CONCLUSIONS
Over the recent years, the shortcomings of WBRT in the treatment of BM from MM have become apparent. At the same time, SRS is evolving as a widely available treatment approach that provides effective and safe treatment of BM at acceptable levels of toxicity. While initial SRS and surgery offer different treatment modalities for the same patient population, this study suggests that surgery remains the cornerstone in the treatment of patients with large BM from MM, as it has been shown to lead to excellent tumor control rates and potentially improved survival. However, technical advances of SRS and earlier detection of small and even clinically asymptomatic lesions make primary SRS increasingly an attractive choice for the management of patients with single or very few BMs. Our study and others indicate that initial surgery may play a crucial role to the addition of SRS for improving local control, neurological status and even OS in patients with symptomatic BMs from MM. This deserves further study either through a randomized controlled trial or through nonrandomized parallel group studies or studies of controlled interrupted-time series.
References
1. Amer MH, Al-Sarraf M, Baker LH, Vaitkevicius VK. Malignant melanoma and central nervous system metastases. Cancer. 1978. 42: 660-8
2. Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004. 363: 1665-72
3. Bernard ME, Wegner RE, Reineman K, Heron DE, Kirkwood J, Burton SA. Linear accelerator based stereotactic radiosurgery for melanoma brain metastases. J Cancer Res Ther. 2012. 8: 215-21
4. Bindal AK, Bindal RK, Hess KR, Shiu A, Hassenbusch SJ, Shi WM. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg. 1996. 84: 748-54
5. Bindal RK, Sawaya R, Leavens ME, Lee JJ. Surgical treatment of multiple brain metastases. J Neurosurg. 1993. 79: 210-6
6. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009. 10: 1037-44
7. Clarke JW, Register S, McGregor JM, Grecula JC, Mayr NA, Wang JZ. Stereotactic radiosurgery with or without whole brain radiotherapy for patients with a single radioresistant brain metastasis. Am J Clin Oncol. 2010. 33: 70-4
8. DiLuna ML, King JT, Knisely JP, Chiang VL. Prognostic factors for survival after stereotactic radiosurgery vary with the number of cerebral metastases. Cancer. 2007. 109: 135-45
9. Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist. 2007. 12: 884-98
10. Fife KM, Colman MH, Stevens GN, Firth IC, Moon D, Shannon KF. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004. 22: 1293-300
11. Fogarty G, Morton RL, Vardy J, Nowak AK, Mandel C, Forder PM. Whole brain radiotherapy after local treatment of brain metastases in melanoma patients - a randomised phase III trial. BMC Cancer. 2011. 11: 142-
12. Gaspar L, Scott C, Rotman M. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997. 37: 745-51
13. Gaudy-Marqueste C, Regis JM, Muracciole X, Laurans R, Richard MA, Bonerandi JJ. Gamma-Knife radiosurgery in the management of melanoma patients with brain metastases: A series of 106 patients without whole-brain radiotherapy. Int J Radiat Oncol Biol Phys. 2006. 65: 809-16
14. Gibney GT, Forsyth PA, Sondak VK.editors. Melanoma in the brain: Biology and therapeutic options. Melanoma Res. 2012. 22: 177-83
15. Hanson PW, Elaimy AL, Lamoreaux WT, Demakas JJ, Fairbanks RK, Mackay AR. A concise review of the efficacy of stereotactic radiosurgery in the management of melanoma and renal cell carcinoma brain metastases. World J Surg Oncology. 10: 176-
16. Hara W, Tran P, Li G, Su Z, Puataweepong P, Adler JR. Cyberknife for brain metastases of malignant melanoma and renal cell carcinoma. Neurosurgery. 2009. 64(2 Suppl): A26-32
17. Jenkinson MD, Haylock B. Management of cerebral metastasis: Evidence-based approach for surgery, stereotactic radiosurgery and radiotherapy. Eur J Cancer. 2011. 47: 649-55
18. Jensen RL, Shrieve AF, Samlowski W, Shrieve DC. Outcomes of patients with brain metastases from malignant melanoma and renal cell carcinoma after primary stereotactic radiosurgery. Clin Neurosurg. 2008. 55: 150-9
19. Khan MK, Khan N, Almasan A, Macklis R. Future of radiation therapy for malignant melanoma in an era of newer, more effective biological agents. Oncol Targets Ther. 2011. 4: 137-48
20. Koc M, McGregor J, Grecula J, Bauer CJ, Gupta N, Gahbauer RA. Gamma Knife radiosurgery for intracranial metastatic melanoma: An analysis of survival and prognostic factors. J Neurooncol. 2005. 71: 307-13
21. Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011. 29: 134-41
22. Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ. Meta-Analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008. 26: 527-34
23. Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI. Identification of prognostic factors in patients with brain metastases: A review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999. 43: 795-803
24. Lavine SD, Petrovich Z, Cohen-Gadol AA. Gamma knife radiosurgery for metastatic melanoma: An analysis of survival, outcome, and complications. Neurosurgery. 1999. 44: 59-64
25. Liew DN, Kano H, Kondziolka D, Mathieu D, Niranjan A, Flickinger JC. Outcome predictors of Gamma Knife surgery for melanoma brain metastases.Clinical article. J Neurosurg. 2011. 114: 769-79
26. Majer M, Samlowski WE. Management of metastatic melanoma patients with brain metastases. Curr Oncol Rep. 2007. 9: 411-6
27. Mori Y, Kondziolka D, Flickinger JC, Krikwood JM, Agarwala S, Lunsford LD. Stereotactic radiosurgery for cerebral metastatic melanoma: Factors affecting local disease control and survival. Int J Radiat Oncol Biol Phys. 1998. 42: 581-9
28. Mut M. Surgical treatment of brain metastasis: A review. Clin Neurol Neurosurg. 2012. 114: 1-8
29. Nieder C, Andratschke NH, Spanne O, Geinitz H, Grosu AL. Does overall treatment time impact on survival after whole-brain radiotherapy for brain metastases?. Clin Transl Oncol Off Publ Fed Spanish Oncol Soc Natl Cancer Inst Mex. 2011. 13: 885-8
30. O′Neill BP, Iturria NJ, Link MJ, Pollock BE, Ballman KV, O′Fallon JR. A Comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003. 55: 1169-76
31. Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003. 29: 533-40
32. Patchell R, Tibbs P. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990. 322: 494-500
33. Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ.editors. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998. 280: 1485-9
34. Powell JW, Chung CT, Shah HR, Canute GW, Hodge CJ, Bassano DA. Gamma Knife surgery in the management of radioresistant brain metastases in high-risk patients with melanoma, renal cell carcinoma, and sarcoma. J Neurosurg. 2008. 109 Suppl: 122-8
35. Selek U, Chang EL, Hassenbusch SJ, Shiu AS, Lang FF, Allen P. Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Radiat Oncol Biol Phys. 2004. 59: 1097-106
36. Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. Cancer J Clin. 2012. 62: 10-29
37. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012. 30: 419-25
38. Tsao MN, Lloyd NS, Wong RK, Rakovitch E, Chow E, Laperriere N. Radiotherapeutic management of brain metastases: A systematic review and meta-analysis. Cancer Treat Rev. 2005. 31: 256-73
39. Weltman E, Salvajoli JV, Brandt RA, de Morais Hanriot R, Prisco FE, Cruz JC. Radiosurgery for brain metastases: A score index for predicting prognosis. Int J Radiat Oncol Biol Phys. 2000. 46: 1155-61
40. Yu C, Chen JC, Apuzzo ML, O′Day S, Giannotta SL, Weber JS.editors. Metastatic melanoma to the brain: Prognostic factors after Gamma Knife radiosrugery. Int J Radiat Oncol Biol Phys. 2002. 52: 1277-87