- Department of Neurosurgery, Saitama Medical University International Medical Center, Yamane, Hidaka, Japan.
- Department of Critical Care and Emergency, Saitama Medical University International Medical Center, Yamane, Hidaka, Japan.
Correspondence Address:
Ryosuke Tsuchiya, Department of Neurosurgery, Saitama Medical University International Medical Center, Hidaka, Saitama, Japan.
DOI:10.25259/SNI_544_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ryosuke Tsuchiya1, Hidetoshi Ooigawa1, Tatsuki Kimura1, Shinya Tabata1, Takuma Maeda1, Hiroki Sato1, Kaima Suzuki1, Yasuhiro Ohara2, Yoshitaka Ooya2, Manabu Nemoto2, Hiroki Kurita1. Study of certain easily available biochemical markers in prognostication in severe traumatic brain injury requiring surgery. 01-Dec-2023;14:410
How to cite this URL: Ryosuke Tsuchiya1, Hidetoshi Ooigawa1, Tatsuki Kimura1, Shinya Tabata1, Takuma Maeda1, Hiroki Sato1, Kaima Suzuki1, Yasuhiro Ohara2, Yoshitaka Ooya2, Manabu Nemoto2, Hiroki Kurita1. Study of certain easily available biochemical markers in prognostication in severe traumatic brain injury requiring surgery. 01-Dec-2023;14:410. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12655
Abstract
Background: This study aimed to identify easily available prognostic factors in severe traumatic brain injury (TBI) patients undergoing craniotomy.
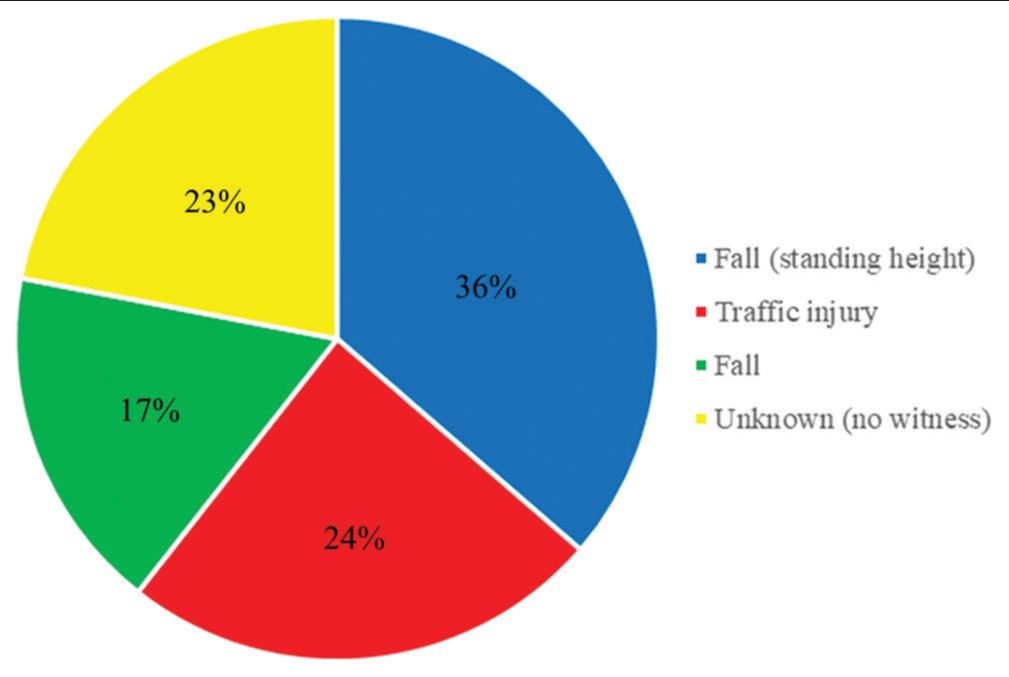
Methods: We retrospectively analyzed the clinical characteristics (age, sex, Glasgow coma scale score, cause of TBI, and oral antithrombotic drug use), laboratory parameters (hemoglobin, sodium, C-reactive protein, D-dimer, activated partial thromboplastin time, prothrombin time-international normalized ratio, and glucose-potassium [GP] ratio), and neuroradiological findings of 132 patients who underwent craniotomy for severe TBI in our hospital between January 2015 and December 2021. The patients were divided into two groups: Those with fatal clinical outcomes and those with non-fatal clinical outcomes, and compared between the two groups.
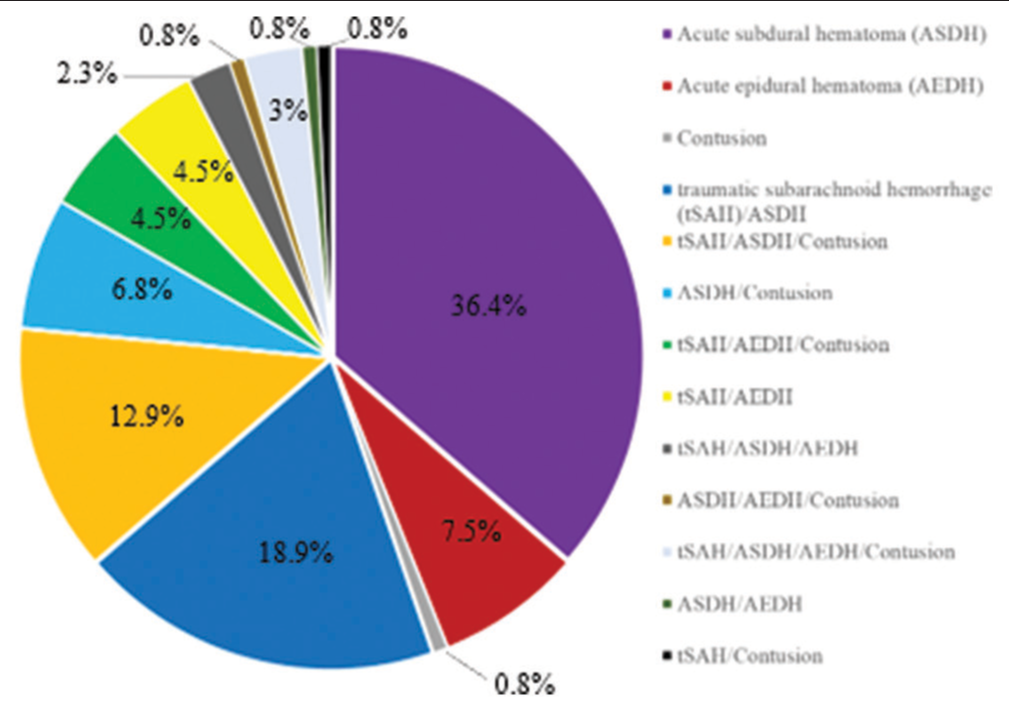
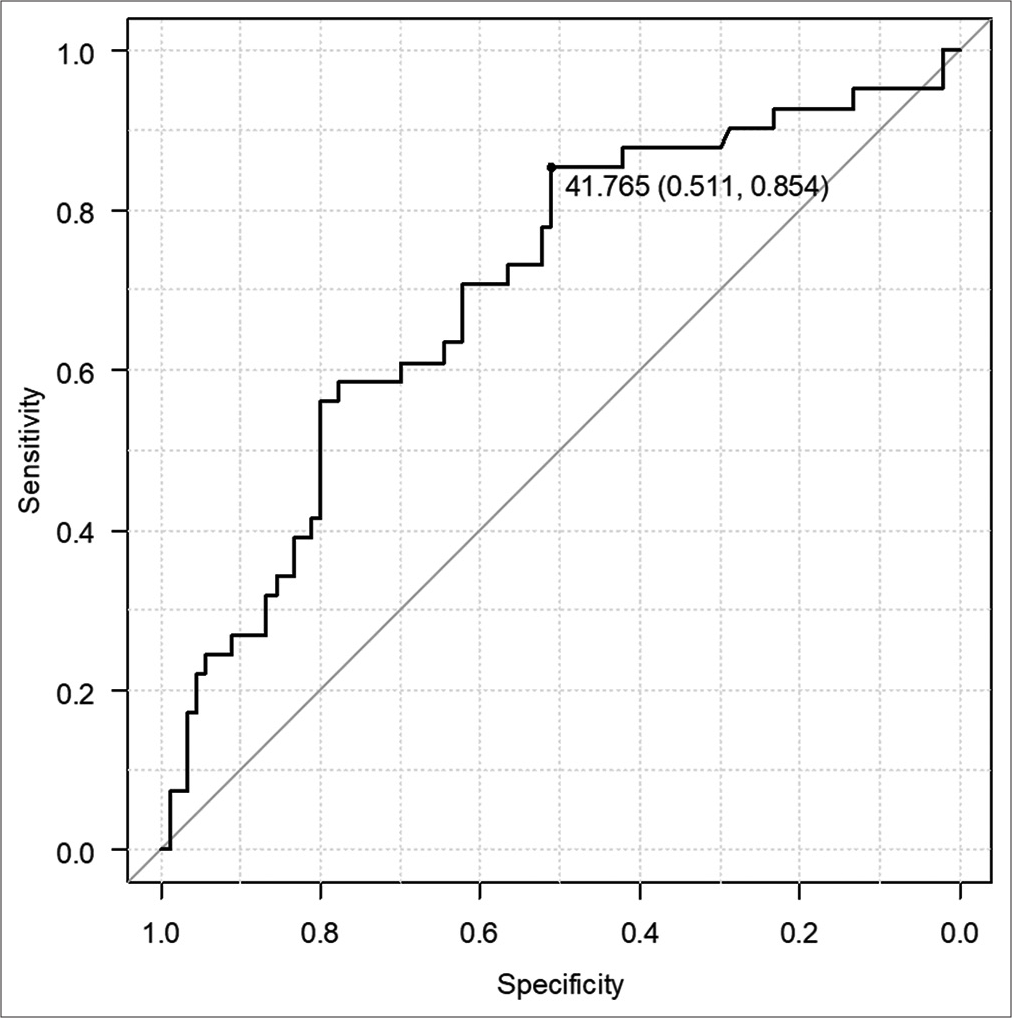
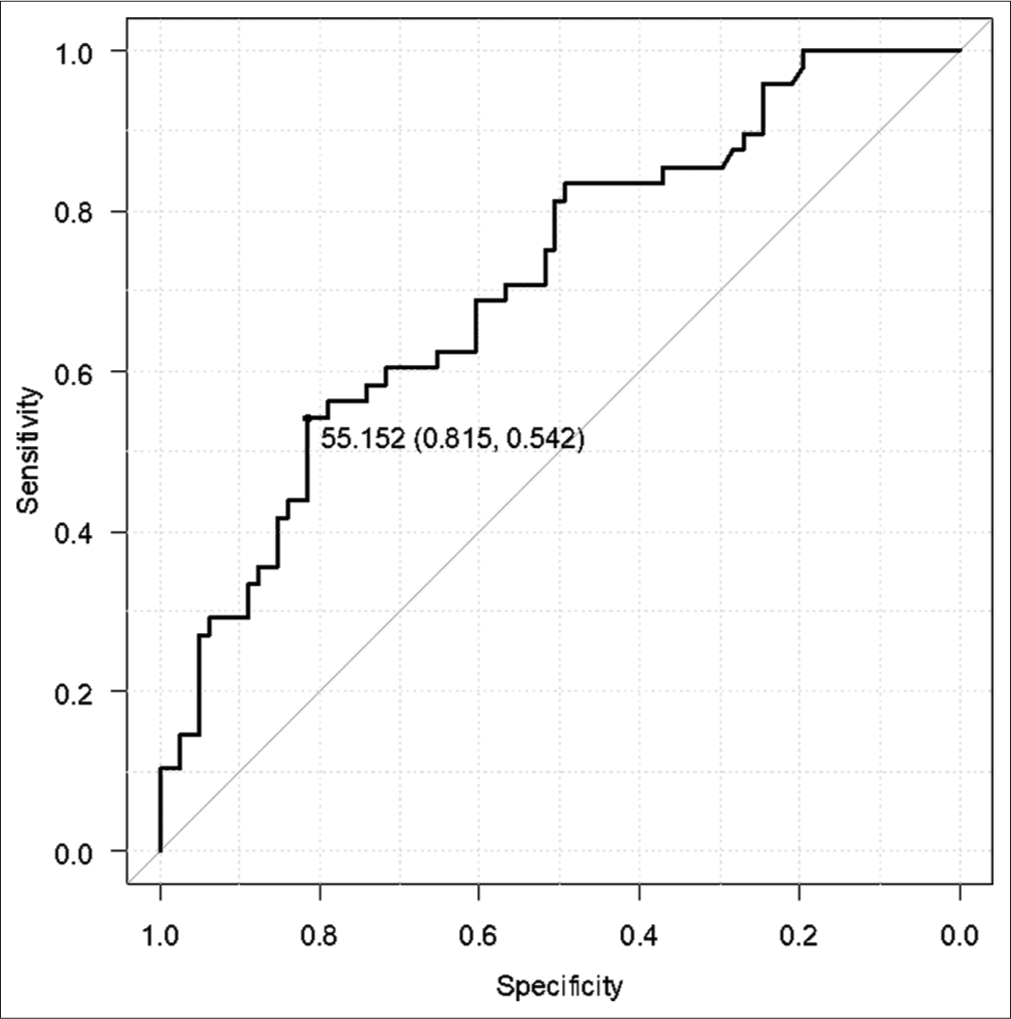
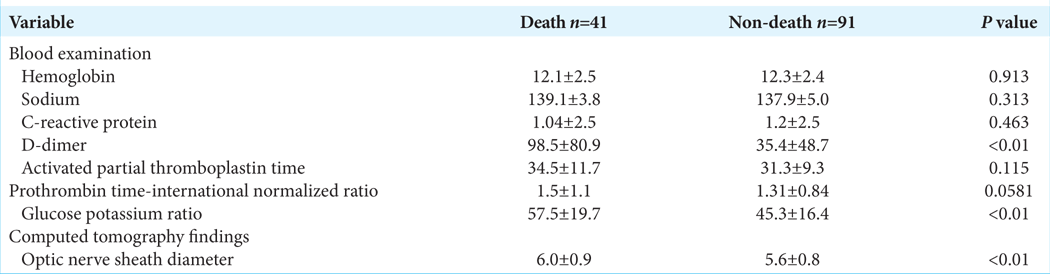
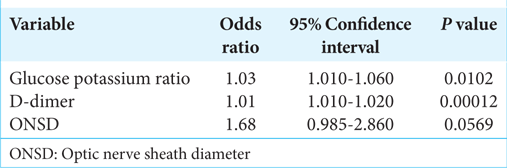
Results: The patients comprised 79 (59.8%) male and 53 (40.2%) female patients. Their mean age was 67 ± 17 years (range, 16–94 years). Computed tomography revealed acute subdural hematoma in 108 (81.8%) patients, acute epidural hematoma in 31 (23.5%), traumatic brain contusion in 39 (29.5%), and traumatic subarachnoid hemorrhage in 62 (47.0%). All 132 patients underwent craniotomy, and 41 eventually died. There were significant differences in the D-dimer, GP ratio, and optic nerve sheath diameter between the groups (all P P P 42 was the optimal cutoff value for the prediction of a fatal outcome of TBI (sensitivity, 85.4%; specificity, 51.1%).
Conclusion: The GP ratio and D-dimer were significantly associated with poor outcomes of TBI. A GP ratio of >42 could be a predictor of a fatal outcome of TBI.
Keywords: Neurosurgery, Prognostic factors, Traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) remains a common cause of death and disability worldwide.[
MATERIALS AND METHODS
Study design and patient selection
The study had a retrospective and observational single-center design and included 153 patients with TBI who underwent open surgery between January 2015 and December 2021. Patients with multiple trauma (moderate or severe trauma with an abbreviated injury scale score >3 at sites other than the brain, n = 15) and those who died from systemic disease (n = 6) were excluded from the study. Finally, 132 patients were enrolled [
Data collection and diagnostic modalities
We collected the following patient data on arrival in the emergency room: age, sex, GCS score, cause of TBI, oral antithrombotic drug use, blood tests including hemoglobin (g/dL), sodium (mEq/L), C-reactive protein (mg/dL), D-dimer (mg/dL), activated partial thromboplastin time(s), prothrombin time-international normalized ratio, and glucose and potassium. The specific glucose-potassium (GP) ratio is calculated in peripheral blood measurements and has been reported to be a useful biomarker of subarachnoid hemorrhage.[
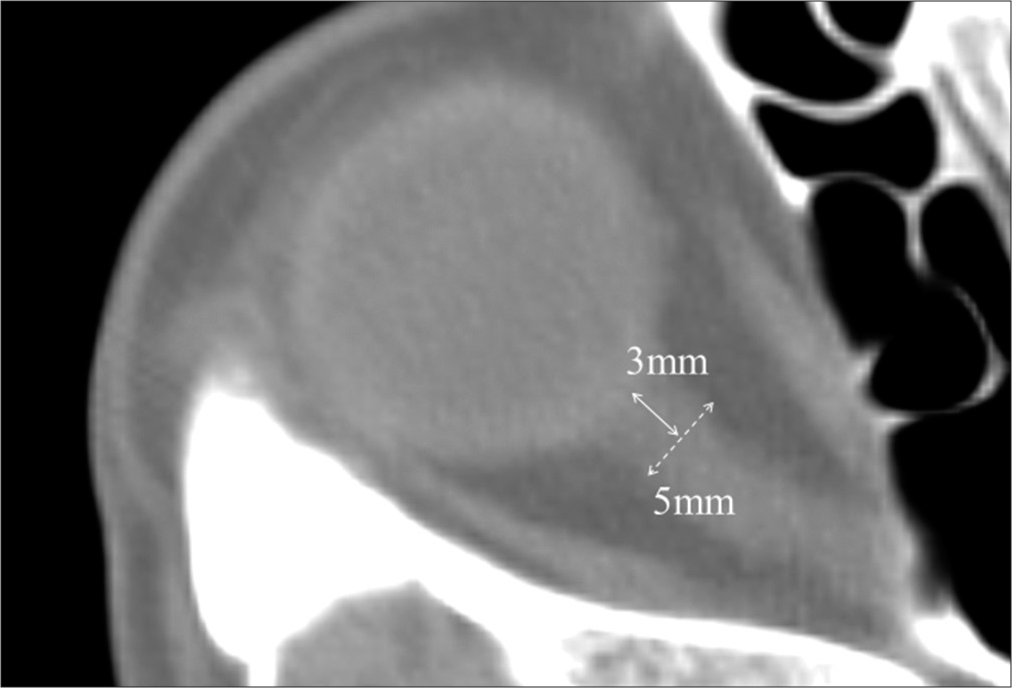
Findings on CT were reviewed for patients who had scans before, immediately after, and one-day post-surgery. We confirmed the diagnosis of TBI and assessed the CT images for the occurrence of injury-related cerebral infarction. Most cases of injury-related cerebral infarction could not be detected by preoperative CT alone and required confirmation on postsurgical CT images. Optic nerve sheath diameter (ONSD) was measured on the preoperative CT images. ONSD is easily obtained in the initial emergency care setting, and it has been reported that a diameter wider than 5 mm indicates increasing intracranial pressure (ICP).[
Surgical procedures
The indication for surgery was based on the Japanese Neurotrauma Society guidelines.[
Statistical analysis
Quantitative variables were expressed as the mean ± standard deviation or the median (range) as appropriate. Normally distributed variables were compared using the Student’s t-test, and non-normally distributed variables using the Mann–Whitney U -test. Multivariable logistic regression analysis was performed on factors that showed a significant between-group difference in univariate analysis. P < 0.05 was considered statistically significant. The optimal cutoff value on the receiver-operating characteristic (ROC) curve was determined based on the Youden index. All statistical analyses were performed with Easy R (Saitama Medical Center, Jichi Medical University, Saitama, Japan).[
RESULTS
The clinical characteristics of the 132 patients are shown in
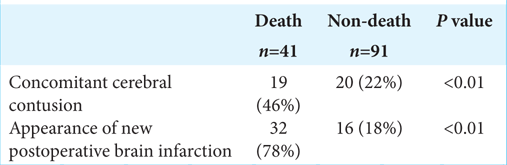
CT revealed acute subdural hematoma (ASDH) in 108 patients (81.8%), acute epidural hematoma (AEDH) in 31 (23.5%), traumatic brain contusion in 39 (29.5%), and traumatic subarachnoid hemorrhage in 62 (47%). ASDH complicated other TBI in 56% of cases, as AEDH complicated in 68% of cases [
DISCUSSION
There have been several studies of prognostic factors and scales for TBI, but none have identified any factors that definitely predict the prognosis. In this study, we focused on clinical biochemical data, which can be collected easily from a peripheral blood sample or on CT scans other than prognostic factors in several scales.[
Epinephrine is well known to be released under stressful conditions, including TBI, and induces an increase in potassium and glucose.[
We also identified a relationship between the GP ratio and injury-related cerebral infarction. A GP ratio of 55 was the cutoff value for injury-related cerebral infarction. S100B, GFAP, neuron-specific enolase, and UCH-L1 have been reported to be specific biomarkers after TBI. Joseph et al.[
As previously reported,[
This study has several limitations. First, it only included Japanese patients who underwent open neurosurgery at a single center. Second, the analyzed data were obtained from patients undergoing open neurosurgery that several neurosurgeons performed. The guidelines were followed regarding surgical indications, but it was left to the individual surgeon whether to perform external decompression or ICP sensor insertion, which might have affected the outcome. Third, the results in terms of the GP ratio might have been affected by the inclusion of patients with diabetes and chronic renal impairment, both of which affect potassium and glucose levels.
CONCLUSION
In this study, there was a significant relationship between the GP ratio and D-dimer level and the survival outcome in patients with TBI. For the GP ratio, the cutoff value was 42 for the prediction of death and 55 for the prediction of injury-related cerebral infarction. Our findings suggest that the GP ratio should be measured in patients with TBI requiring open neurosurgery.
Ethical approval
The author(s) declare that they have taken the ethical approval from the IEC (approval number 2022–051).
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
I want to thank all the authors. Hidetoshi Ooigawa and Hiroki Kurita substantially contributed to the study conceptualization. Hidetoshi Ooigawa significantly contributed to data analysis, writing, and interpretation. All authors critically reviewed and revised the manuscript and approved the final version for submission.
References
1. Abdesselam OB, Vally J, Adem C, Foglietti MJ, Beaudeux JL. Reference values for serum S-100B protein depend on the race of individuals. Clin Chem. 2003. 49: 836-7
2. Cheng F, Yuan Q, Yang J, Wang W, Liu H. The prognostic value of serum neuron-specific enolase in traumatic brain injury: Systematic review and meta-analysis. PLoS One. 2014. 9: e106680
3. De Kruijk JR, Leffers P, Menheere PP, Meerhoff S, Twijnstra A. S-100B and neuron-specific enolase in serum of mild traumatic brain injury patients. A comparison with health controls. Acta Neurol Scand. 2001. 103: 175-9
4. Fujiki Y, Matano F, Mizunari T, Murai Y, Tateyama K, Koketsu K. Serum glucose/potassium ratio as a clinical risk factor for aneurysmal subarachnoid hemorrhage. J Neurosurg. 2018. 129: 870-5
5. Iaccarino C, Carretta A, Nicolosi F, Morselli C. Epidemiology of severe traumatic brain injury. J Neurosurg Sci. 2018. 62: 535-41
6. Joseph B, Pandit V, Zangbar B, Kulvatunyou N, Khalil M, Tang A. Secondary brain injury in trauma patients: The effects of remote ischemic conditioning. J Trauma Acute Care Surg. 2015. 78: 698-703
7. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013. 48: 452-8
8. Kim DY, Kim SY, Hong DY, Sung BY, Lee S, Paik JH. Comparison of ultrasonography and computed tomography for measuring optic nerve sheath diameter for the detection of elevated intracranial pressure. Clin Neurol Neurosurg. 2021. 204: 106609
9. Lee EJ, Hung YC, Wang LC, Chung KC, Chen HH. Factors influencing the functional outcome of patients with acute epidural hematomas: Analysis of 200 patients undergoing surgery. J Trauma. 1998. 45: 946-52
10. Lim M, Linton RA, Band DM. Continuous intravascular monitoring of epinephrine-induced changes in plasma potassium. Anesthesiology. 1982. 57: 272-8
11. Lu Y, Ma X, Zhou X, Wang Y. The association between serum glucose to potassium ratio on admission and short-term mortality in ischemic stroke patients. Sci Rep. 2022. 12: 8233
12. Maas AI, Steyerberg EW, Butcher I, Dammers R, Lu J, Marmarou A. Prognostic value of computerized tomography scan characteristics in traumatic brain injury: Results from the IMPACT study. J Neurotrauma. 2007. 24: 303-14
13. Mahan MY, Thorpe M, Ahmadi A, Abdallah T, Casey H, Sturtevant D. Glial fibrillary acidic protein (GFAP) outperforms S100 calcium-binding protein B (S100B) and ubiquitin C-terminal hydrolase L1 (UCH-L1) as predictor for positive computed tomography of the head in trauma subjects. World Neurosurg. 2019. 128: e434-44
14. Matano F, Fujiki Y, Mizunari T, Koketsu K, Tamaki T, Murai Y. Serum glucose and potassium ratio as risk factors for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2019. 28: 1951-7
15. Perel P, Arango M, Clayton T, Edwards P, Komolafe E. Predicting outcome after traumatic brain injury: Practical prognostic models based on large cohort of international patients. BMJ. 2008. 336: 425-9
16. Murray GD, Brennan PM, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 2: Graphical presentation of probabilities. J Neurosurg. 2018. 128: 1621-34
17. Ramesh VG, Thirumaran KP, Raja MC. A new scale for prognostication in head injury. J Clin Neurosci. 2008. 15: 1110-3
18. Shibata A, Matano F, Saito N, Fujiki Y, Matsumoto H, Mizunari T. Serum glucose-to-potassium ratio as a prognostic predictor for severe traumatic brain injury. J Nippon Med Sch. 2021. 88: 342-6
19. Takayama Y, Yokota H, Sato H, Naoe Y, Araki T. Pathophysiology, mortality, treatment of acute phase of haemostatic disorders of traumatic brain injury. Jpn J Neurosurg (Tokyo). 2013. 22: 837-41
20. Wu XY, Zhuang YK, Cai Y, Dong XQ, Wang KY, Du Q. Serum glucose and potassium ratio as a predictive factor for prognosis of acute intracerebral hemorrhage. J Int Med Res. 2021. 49: 1-12
21. Yatsushige H, Maeda T, Takasato Y, editors. Traumatic brain injury treatment and management guidelines. Japan: Igaku Shoin; 2019. p. 105-14
22. Zhou J, Yang CS, Shen LJ, Lv QW, Xu QC. Usefulness of serum glucose and potassium ratio as a predictor for 30-day death among patients with severe traumatic brain injury. Clin Chim Acta. 2020. 506: 166-71