- Department of Neurosurgery, RWTH Aachen University Hospital, Aachen, Germany,
- Department of Neurosurgery, University of Helsinki and Helsinki University Hospital,
- Department of Neurosurgery, Neuromed Campus, Kepler University Hospital, Linz, Austria,
- Department of Neurosurgery, University Hospital Helsinki, Helsinki, Finland.
Correspondence Address:
Michael Veldeman, Department of Neurosurgery, RWTH Aachen University Hospital, Aachen, Germany.
DOI:10.25259/SNI_1012_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Michael Veldeman1,2, Tobias Rossmann2,3, Nuutti Vartiainen2, Mika Niemela4. Subtemporal approach for cavernous sinus meningiomas – Simple and effective. 20-Jan-2023;14:16
How to cite this URL: Michael Veldeman1,2, Tobias Rossmann2,3, Nuutti Vartiainen2, Mika Niemela4. Subtemporal approach for cavernous sinus meningiomas – Simple and effective. 20-Jan-2023;14:16. Available from: https://surgicalneurologyint.com/surgicalint-articles/12115/
Abstract
Background: Over the past few decades, there has been a paradigm shift in treatment strategy for cavernous sinus meningiomas (CSMs). Preserving neurological function and cranial nerve (CN) decompression have become the primary goal of cases eligible for surgical treatment. Extensive skull base dissection and drilling can be avoided by approaching these lesions through a subtemporal route.
Methods: We describe the subtemporal approach in a step-by-step fashion illustrating its advantages and pitfalls through and illustrative case.
Results: The subtemporal approach to CSMs is a valuable alternative for CN decompression and maximal safe resection. We describe the technique in comparison to classical skull base approaches. Although rare, recurrence after adjuvant maximal radiation is possible leaving reoperation as the only treatment option.
Conclusion: The subtemporal approach offers a less invasive alternative for initial and redo CN decompression and successful symptom control in patients suffering from CSM.
Keywords: Cavernous sinus, Meningioma, Skull base, Subtemporal approach
INTRODUCTION
Cavernous sinus meningioma (CSM) constitutes one of the most challenging skull base meningiomas to treat. Left untreated, CSM can cause ophthalmoplegia, visual loss, and endocrine disturbances. Due to the encasement of the carotid artery and cranial nerves (CNs) III, IV, V1, V2, and VI, the cavernous sinus was coined the “anatomical jewel box” by Dwight Parkinson.[
The resectability of CSM depends mainly on the degree of internal carotid artery encasement similarly to Knosp’s grading for pituitary adenoma.[
CLASSICAL APPROACHES
A frontotemporal/pterional-type of approach with or without orbitozygomatic osteotomy is typically used for CSMs. Extensive extradural dissection by cutting and dividing the meningo-orbital band, drilling of the anterior clinoid and optic strut, and peeling off the temporal dura from the lateral cavernous sinus wall is usually performed.[
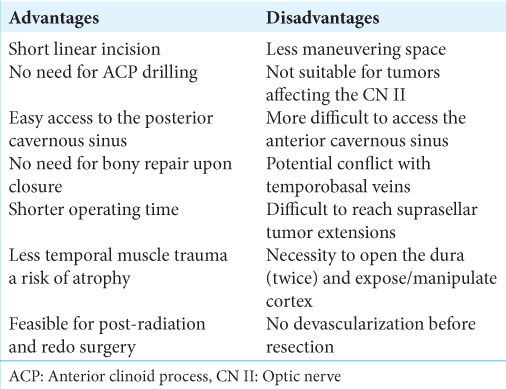
THE SUBTEMPORAL APPROACH
Approaching the cavernous sinus subtemporally eliminates the need for extensive drilling and provides a straight unobstructed route to the lateral wall of the cavernous sinus.
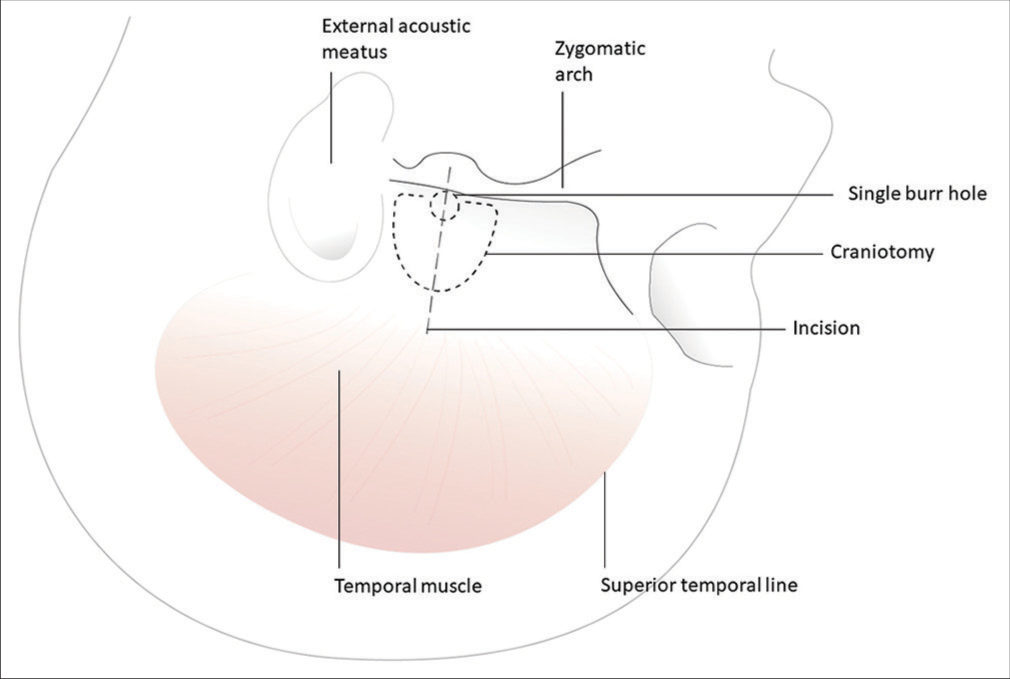
Positioning and craniotomy
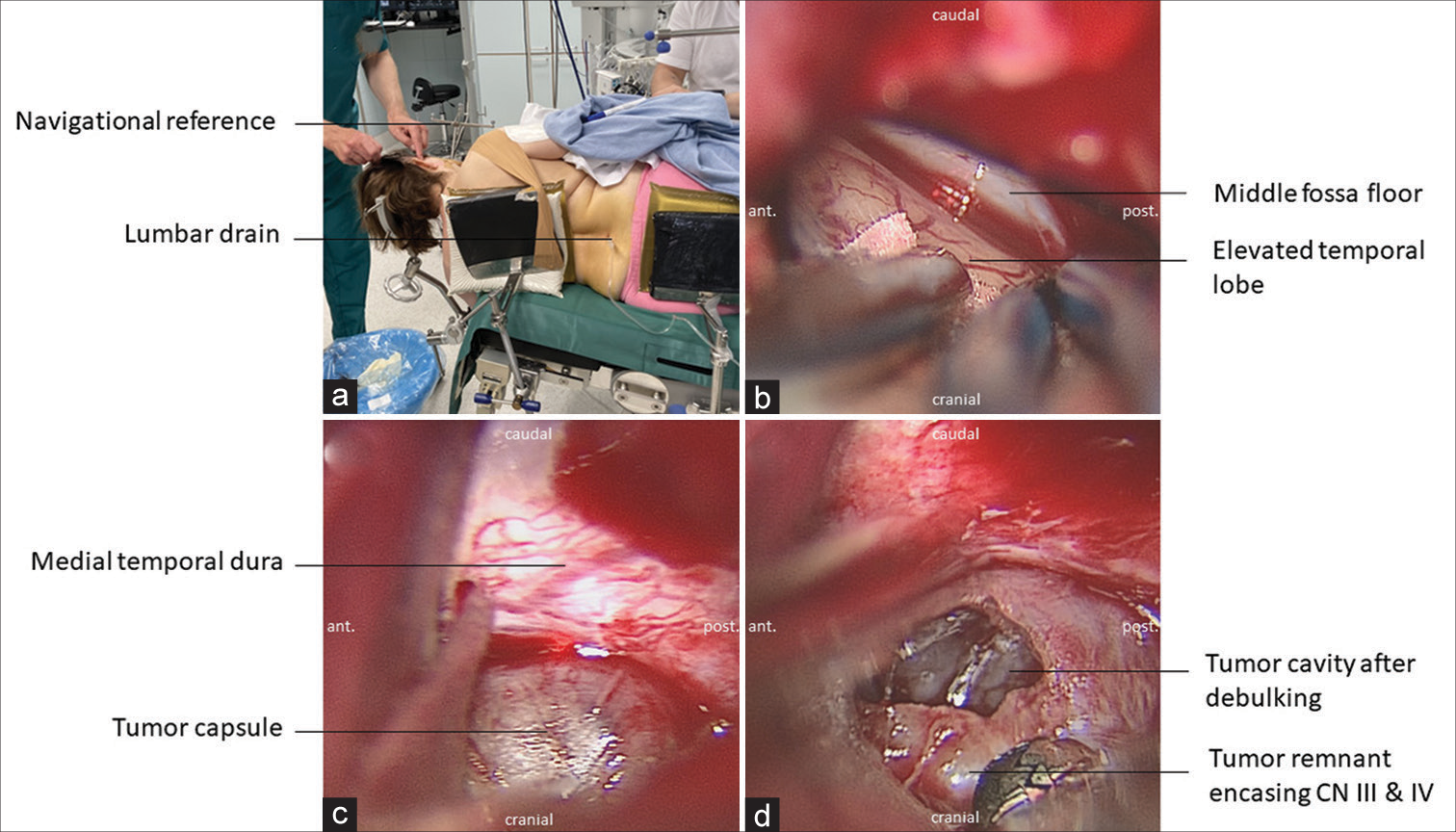
The patient is placed in a park-bench position and to achieve adequate brain relaxation, a lumbar drain is placed, and 50 mL of cerebrospinal fluid (CSF) is removed before incision [
Figure 1:
Surgical steps in cavernous sinus meningioma resection through the subtemporal approach. (a) The patient is placed in a park-bench position allowing 90-° head rotation without compromising venous outflow. A lumbar drain is placed, and 50 mL cerebrospinal fluid is drained before incision. (b) The temporal lobe is elevated exposing the floor of the middle fossa. (c) The tumor is identified after opening of the medial temporal dura. (d) Tumor tissue is either sucked or removed with grasping forceps. A “suckable” tumor consistency is of great benefit to achieve atraumatic tumor removal without cranial nerve injury.
Temporal lobe elevations
Care is taken not to injure the vein of Labbé and the temporal lobe is carefully elevated using dynamic retraction with suction and bipolar only [
Tumor exposure and debulking
The dura overlaying the tumor is incised and the tumor is entered below the anticipated level of the oculomotor and trochlear nerve through the infratrochlear (Parkinson’s) triangle [
ILLUSTRATIVE CASE
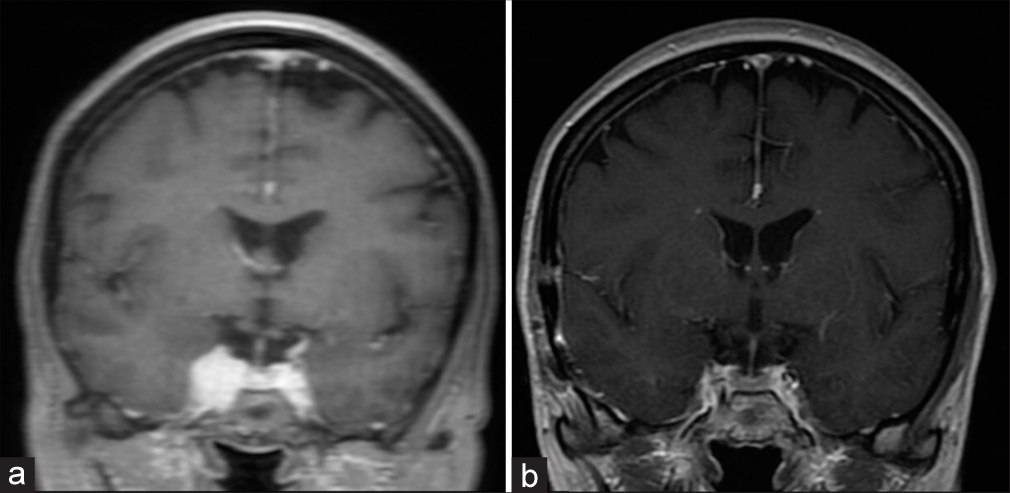
A female patient in her late fifties sought medical advice due to fluctuating mild diplopia. Visual acuity was intact, and a partial CN VI palsy was diagnosed. Magnetic resonance imaging (MRI) revealed a homogenously contrast enhancing lesion of the right cavernous sinus consistent with CSM Hirsch grade II [
Figure 3:
Pre- and postoperative coronal T1 contrast-enhanced magnetic resonance imaging. (a) Initial magnetic resonance (MR)-imaging revealed a homogenously contrast enhancing lesion of the right cavernous sinus consistent with cavernous sinus meningioma. (b) Three months after surgery, MR-imaging revealed no extracavernous residual tumor.
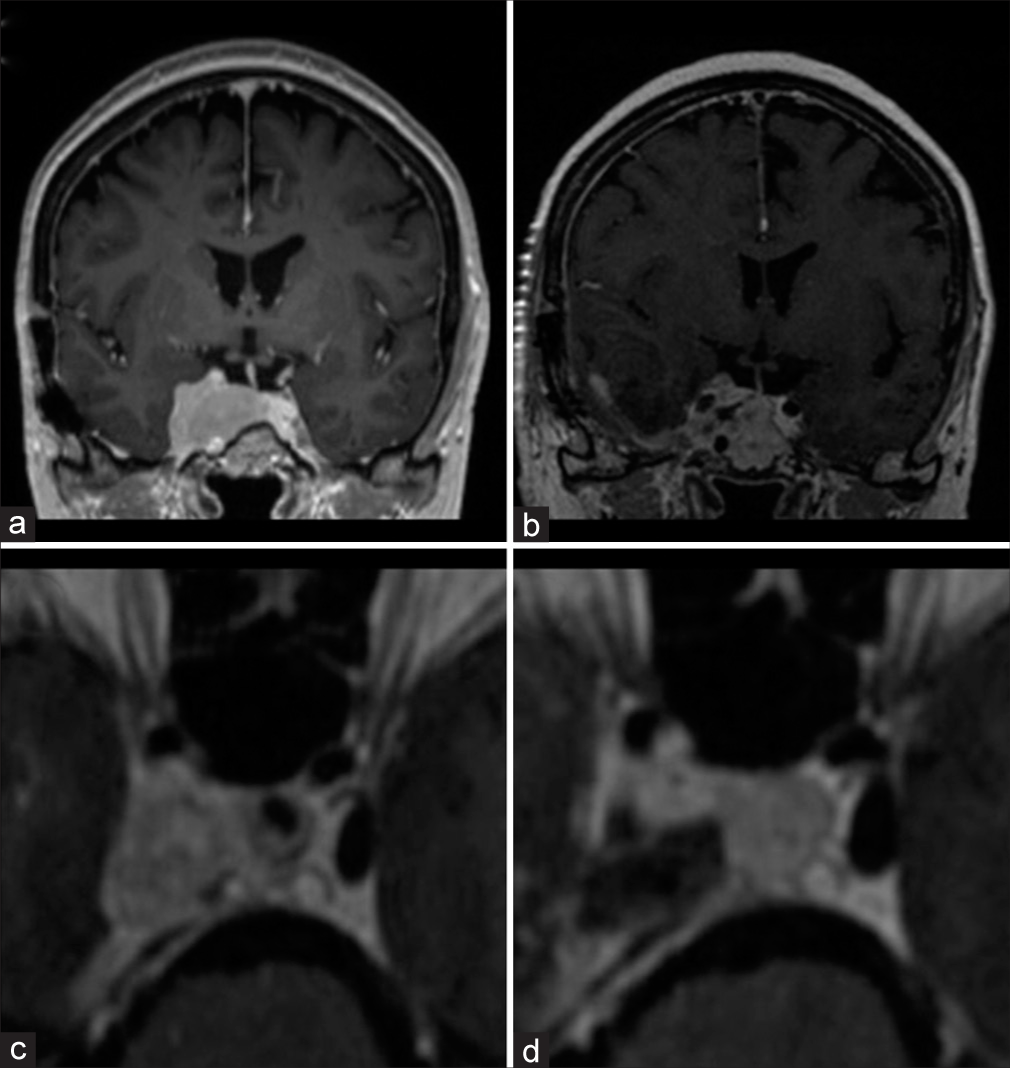
The patient was regularly followed with MRI. Two years later, mild growth of residual intracavernous tumor was documented on imaging, and subsequently, she underwent adjuvant radiotherapy. Fractionated stereotactic irradiation was administered up to 50.4 Gray in 2.8 Gray doses. She remained in regular follow-up, and symptoms of CN VI palsy with diplopia began to reoccur 10 years later. Considerable intra- and extracavernous tumor growth was, now documented on MRI demonstrating a now Hirsch grade III tumor with carotid encasement [
Figure 4:
Pre- and postoperative coronal T1 contrast-enhanced magnetic resonance imaging. (a) Tumor recurrence despite adjuvant radiation after initial resection resulting in CN VI palsy. (b) Early postoperative magnetic resonance-imaging demonstrates internal tumor debulking to treat the cranial nerve compartment syndrome and achieve successful symptom control. (c) Preoperative axial close up T1 contrast-enhanced image of the cavernous sinus region. (d) Postoperative result after subtemporal tumor debulking.
DISCUSSION
During the advent of microsurgical skull base development in the 1990s, the frontiers of what is possible at the level of the cavernous sinus were pushed.[
Alternatives to a frontotemporal approach have been suggested such as a transpetrosal approach[
CONCLUSION
Recurrence of CSMs despite maximal safe resection and adjuvant radiation offers a formidable challenge. The subtemporal approach offers a straightforward, safe, and effective route for the resection of CSM and CN decompression, even in recurrent cases. Sufficient drainage of CSF is key to achieve atraumatic temporal lobe elevation. In the nowadays broadly accepted policy focusing on functional preservation and tumor containment over cure, the subtemporal route provides a less invasive and esthetically superior alternative to classical anterolateral skull base approaches.
DISCLOSURES
Role of the funding source
No funding bodies had any involvement in the preparation of this study or in the decision to submit the paper for publication.
Ethical statement
Ethical approval is not applicable to this technical report. The patient in the illustrative case provided written informed consent.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ajisebutu A, Del Bigio MR, Kazina CJ, West M, Serletis D. Dr. Dwight Parkinson: A Canadian neurosurgical pioneer. J Neurosurg. 2019. 27: 1-8
2. Beer-Furlan A, Priddy BH, Jamshidi AO, Shaikhouni A, Prevedello LM, Filho LD. Improving function in cavernous sinus meningiomas: A modern treatment algorithm. Front Neurol. 2020. 11: 652
3. Corniola MV, Roche PH, Bruneau M, Cavallo LM, Daniel RT, Messerer M. Management of cavernous sinus meningiomas: Consensus statement on behalf of the EANS skull base section. Brain Spine. 2022. 2: 100864
4. DeMonte F, Smith HK, Al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994. 81: 245-51
5. Gozal YM, Alzhrani G, Abou-Al-Shaar H, Azab MA, Walsh MT, Couldwell WT. Outcomes of decompressive surgery for cavernous sinus meningiomas: Long-term follow-up in 50 patients. J Neurosurg. 2020. 132: 380-7
6. Hakuba A, Tanaka K, Suzuki T, Nishimura S. A combined orbitozygomatic infratemporal epidural and subdural approach for lesions involving the entire cavernous sinus. J Neurosurg. 1989. 71: 699-704
7. Hekmatpanah J. Evidence-based treatment of cavernous sinus meningioma. Surg Neurol Int. 2019. 10: 228
8. Hernesniemi J, Ishii K, Niemelä M, Kivipelto L, Fujiki M, Shen H. Subtemporal approach to basilar bifurcation aneurysms: Advanced technique and clinical experience. Acta Neurochir Suppl. 2005. 94: 31-8
9. Hirsch WL, Sekhar LN, Lanzino G, Pomonis S, Sen CN. Meningiomas involving the cavernous sinus: Value of imaging for predicting surgical complications. AJR Am J Roentgenol. 1993. 160: 1083-8
10. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: A magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993. 33: 610-7 discussion 617-8
11. Larson JJ, van Loveren HR, Balko MG, Tew JM. Evidence of meningioma infiltration into cranial nerves: Clinical implications for cavernous sinus meningiomas. J Neurosurg. 1995. 83: 596-9
12. Morisako H, Goto T, Ohata H, Goudihalli SR, Shirosaka K, Ohata K. Safe maximal resection of primary cavernous sinus meningiomas via a minimal anterior and posterior combined transpetrosal approach. Neurosurg Focus. 2018. 44: E11
13. Nanda A, Thakur JD, Sonig A, Missios S. Microsurgical resectability, outcomes, and tumor control in meningiomas occupying the cavernous sinus. J Neurosurg. 2016. 125: 378-92
14. O’Sullivan MG, van Loveren HR, Tew JM. The surgical resectability of meningiomas of the cavernous sinus. Neurosurgery. 1997. 40: 238-44 discussion 245-37
15. Roche PH, Régis J, Dufour H, Fournier HD, Delsanti C, Pellet W. Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurosurg. 2000. 93: 68-73
16. Shaffrey ME, Dolenc VV, Lanzino G, Wolcott WP, Shaffrey CI. Invasion of the internal carotid artery by cavernous sinus meningiomas. Surg Neurol. 1999. 52: 167-71
17. Umekawa M, Shinya Y, Hasegawa H, Shin M, Kawashima M, Katano A. Long-term outcomes of stereotactic radiosurgery for skull base tumors involving the cavernous sinus. J Neurooncol. 2022. 156: 377-86
18. van Loveren HR, Keller JT, el-Kalliny M, Scodary DJ, Tew JM. The dolenc technique for cavernous sinus exploration (cadaveric prosection) Technical note. J Neurosurg. 1991. 74: 837-44
19. Wang B, Li Q, Sun Y, Tong X. Surgical strategy for skull base chordomas: Transnasal midline approach or transcranial lateral approach. J Korean Neurosurg Soc. 2022. 65: 457-68
20. Youssef S, Kim EY, Aziz KM, Hemida S, Keller JT, van Loveren HR. The subtemporal interdural approach to dumbbell-shaped trigeminal schwannomas: Cadaveric prosection. Neurosurgery. 2006. 59: ONS270-7 discussion ONS277-8