- Department of Neuroendovascular Therapy, Saint Luke’s International Hospital, Tokyo, Japan.
Correspondence Address:
Bikei Ryu, Department of Neuroendovascular Therapy, Saint Luke’s International Hospital, Tokyo, Japan.
DOI:10.25259/SNI_413_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tatsuki Mochizuki, Bikei Ryu, Shinsuke Sato, Yasunari Niimi. Successful embolization of ventricular arteriovenous malformation supplied by the choroidal artery: A case report and literature review. 21-Jul-2023;14:254
How to cite this URL: Tatsuki Mochizuki, Bikei Ryu, Shinsuke Sato, Yasunari Niimi. Successful embolization of ventricular arteriovenous malformation supplied by the choroidal artery: A case report and literature review. 21-Jul-2023;14:254. Available from: https://surgicalneurologyint.com/surgicalint-articles/12451/
Abstract
Background: Ventricular arteriovenous malformations (AVMs) are localized in the ventricles and are mainly fed by the anterior choroidal artery (AChoA) and posterior choroidal artery (PChoA). Surgical resection of ventricular AVMs is difficult as the lesions are localized deep in the brain. Therefore, endovascular treatment is expected to treat ventricular AVMs. However, embolization from the AChoA and PChoA carries the risk of ischemic complications. Even though there are some major reports on embolization strategies from the choroidal arteries, embolization of these arteries remains technically challenging. In this article, we report two successful cases of ventricular AVM embolization using AChoA and PChoA.
Case Description: Case 1: A 34-year-old male presented with intraventricular hemorrhage (IVH). Subsequently, ventricular AVM embolization in the anterior horn was performed using n-butyl-2-cyanoacrylate (NBCA) through the AChoA and medial PChoA, and complete obliteration was observed without neurological deterioration. Case 2: A 71-year-old female presented with IVH. Subsequently, ventricular AVM embolization in the lateral ventricle was performed through the AChoA and lateral PChoA with Onyx and NBCA, and partial obliteration was observed without complications. Furthermore, Gamma Knife surgery for residual lesions resulted in complete obliteration.
Conclusion: Embolization through the choroidal arteries for ventricular AVMs is an effective curative or adjunctive treatment.
Keywords: Arteriovenous malformation, Cerebral ventricular hemorrhage, Embolization
INTRODUCTION
Ventricular arteriovenous malformations (AVMs) are rare and account for 5–19% of all cerebral AVMs.[
Here, we report two cases of ventricular AVM which was successfully treated by endovascular embolization through the AChoA and PChoA. We also describe the features of ventricular AVM and the possibility of embolization through the choroidal arteries based on the anatomical features, including a literature review.
CASE DESCRIPTION
Case 1
A 37-year-old male patient with a preceding headache was admitted to our hospital. His consciousness level was a Glasgow Coma Scale (GCS) 13 (E3V4M6), without motor and sensory deficits. Computed tomography (CT) scan of the brain showed IVH and acute obstructive hydrocephalus [
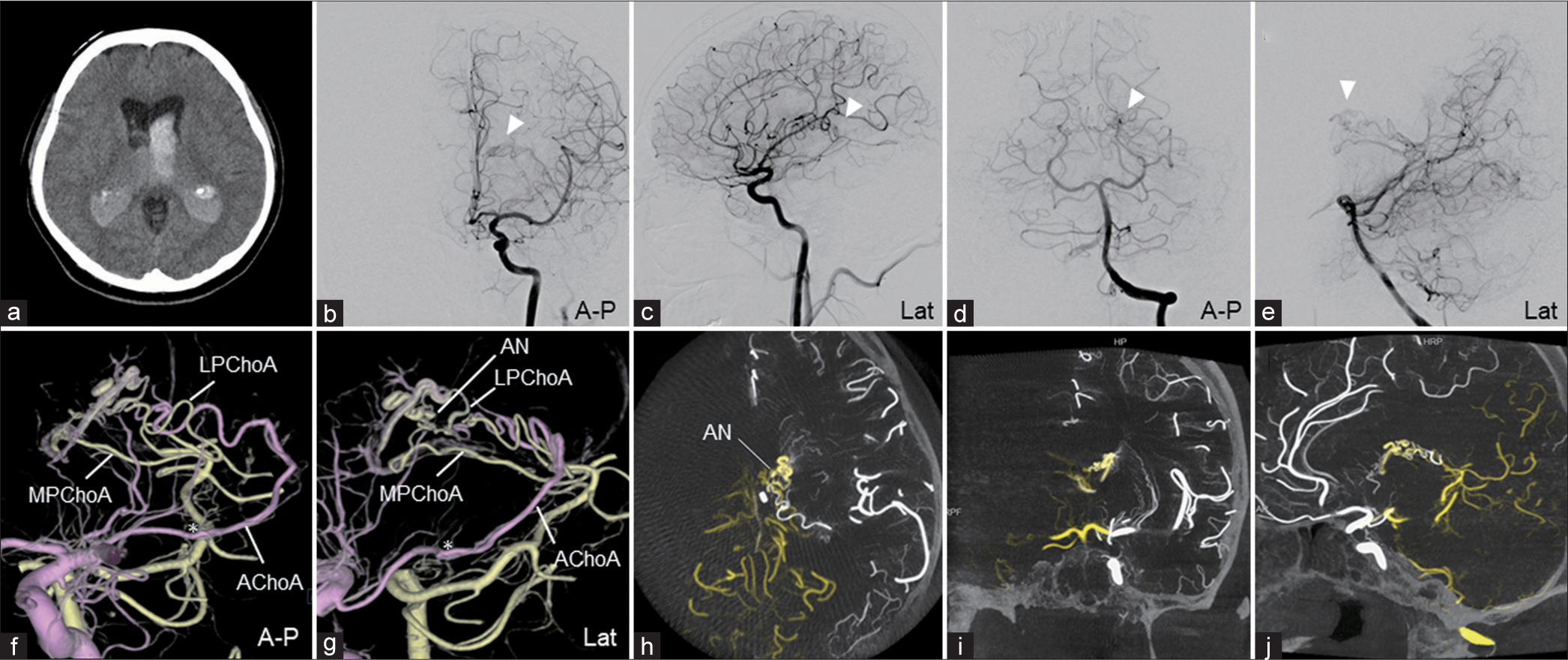
Figure 1:
Preoperative clinical imaging of Case 1 (a) brain computed tomography shows intraventricular hemorrhage and acute hydrocephalus. (b and c) The left ICA angiography shows ventricular AVM fed by the left AChoA and the medial striate artery. (d and e) The left VA angiography shows AVM fed by the left LPChoA and MPChoA. The AVM drains into the internal cerebral vein. White arrowheads show the nidus of the AVM. (f and g) 3D-volume rendering (VR) fusion images (f: A-P, g: lateral view) reconstructed from 3DRA of ICA (pink) and VA (yellow). An intranidal aneurysm was revealed (AN). The asterisk indicates the choroidal point of the AChoA. (h-j) The maximum intensity projection fusion images (h: axial, I coronal, and j: sagittal view) reconstructed from 3D-RA of ICA (white) and VA (yellow). The AVM is located in the body of the caudate. The intranidal aneurysm is within the ventricle. 3D: Three-dimensional, 3D-RA: Three-dimensional rotational angiography, AChoA: Anterior choroidal artery, A-P: Anterior-posterior view, AVM: Arteriovenous malformation, ICA: Internal cerebral artery, Lat: Lateral view, LPChoA: Lateral posterior choroidal artery, MPChoA: Medial posterior choroidal artery, VA: Vertebral artery.
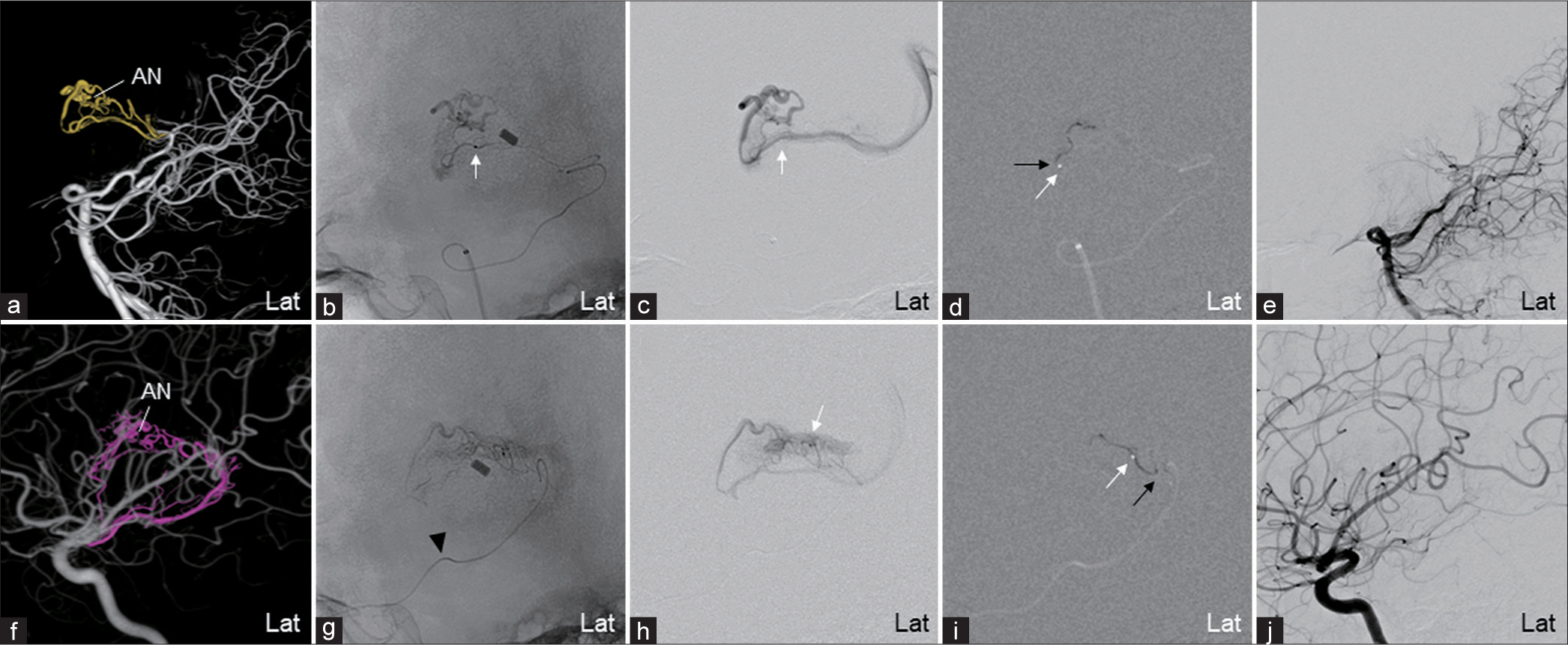
Figure 2:
Embolization of the AVM through the MPChoA and AChoA (Case 1) (a) VR image reconstructed from the 3D-RA of the VA. The AVM is fed by the MPChoA and the LPChoA from the left PCA P2 segment. (b, c) Fluoroscopic and DSA of the selective microcatheter injection. The microcatheter was advanced to the MPChoA plexal segment. (d) Image of the NBCA glue casting injected from the MPChoA. (e) The VA angiography shows the disappearance of AVM from the MPChoA and LPChoA after embolization. (f) VR image reconstructed from the 3D-RA of the ICA. The AVM is fed by the AChoA (pink) and the medial striate artery from the ICA. (g and h) Fluoroscopic and DSA of the microcatheter injection. The microcatheter was advanced to the AChoA plexal segment. Black arrowheads show the choroidal point of the AChoA. (i) Image of the NBCA glue casting injected from the AChoA with slight reflux. (j) ICA angiography shows the disappearance of the AVM after embolization. White and black arrows show the microcatheter’s tip and the reflux of the embolic agent, respectively. There is an aneurysm in the nidus (AN). 3D-RA: Three-dimensional rotational angiography, 3D-VR: Three-dimensional volume rendering, AchoA: Anterior choroidal artery, AVM: Arteriovenous malformation, DSA: Digital subtraction image, ICA: Internal carotid artery, Lat: Lateral view, LPChoA: Lateral posterior choroidal artery, MPChoA: Medial posterior choroidal artery, NBCA: n-butyl-2-cyanoacrylate, PCA: Posterior cerebral artery, PChoA: Posterior choroidal artery, VA: vertebral artery.
Case 2
A 71-year-old comatose female patient was transported to the former hospital. Her consciousness level was a GCS score of 6 (E1V1M4). A brain CT scan showed IVH and acute obstructive hydrocephalus [
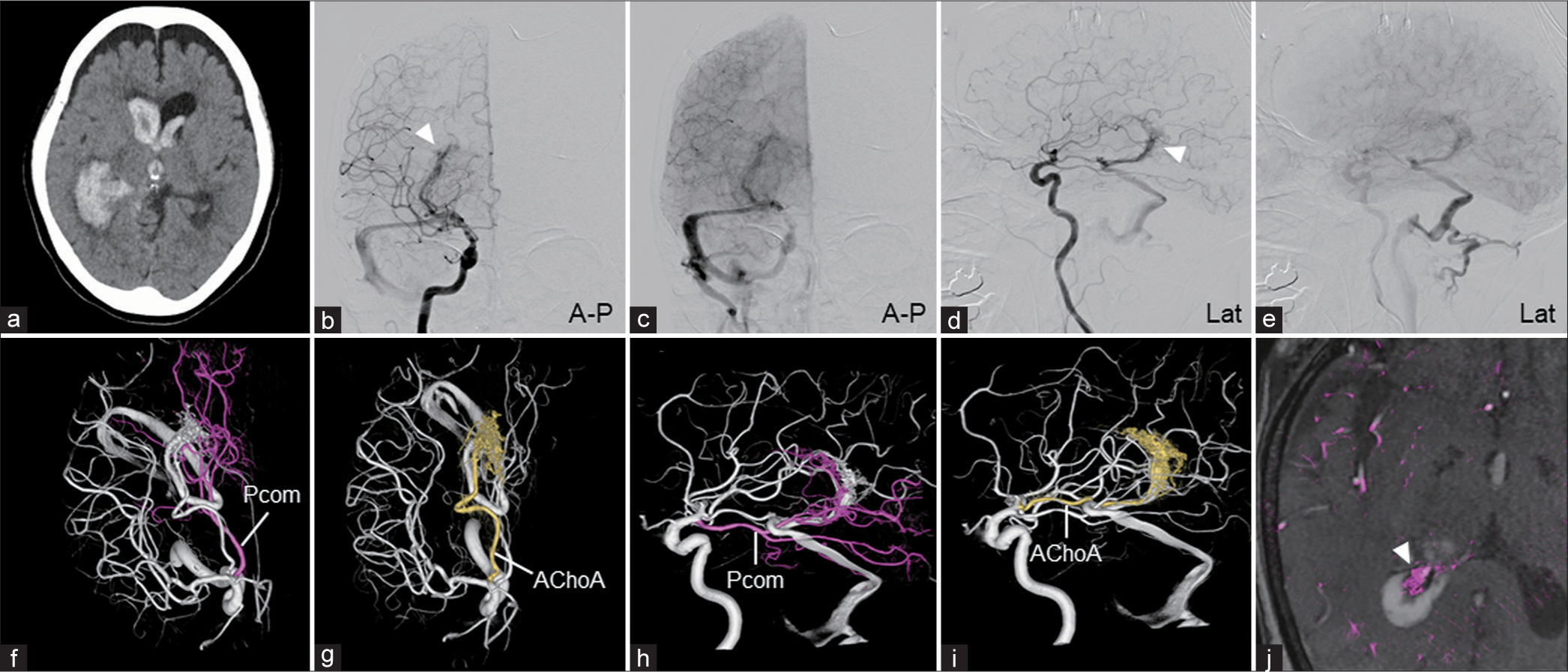
Figure 3:
Preoperative clinical imaging of Case 2 (a) brain computed tomography shows intraventricular hemorrhage and acute hydrocephalus. (b-e) Right ICA angiography shows the AVM fed by AChoA and LPChoA, draining into the inferior ventricular vein to the basal vein of the rosenthal, tentorial sinus, and transverse-sigmoid sinus junction. (f-i) 3D-VR images reconstructed from the ICA (f and g: Cranial, h and i: Lateral view) shows the AVM fed by LPChoA originating from the Pcom (pink) and AChoA (yellow). (j) Fusion image of 3D-RA of ICA and time-of-flight magnetic resonance angiography shows the AVM located within the trigone of the right ventricle. White arrowheads show the nidus of the AVM. 3D-RA: Three-dimensional rotational angiography, 3D-VR: Three-dimensional volume rendering, A-P: Anterior-posterior view, AChoA: Anterior choroidal artery, AVM: Arteriovenous malformation, ICA: Internal carotid artery, Lat: Lateral view, LPChoA: Lateral posterior choroidal artery, Pcom: Posterior communicating artery.
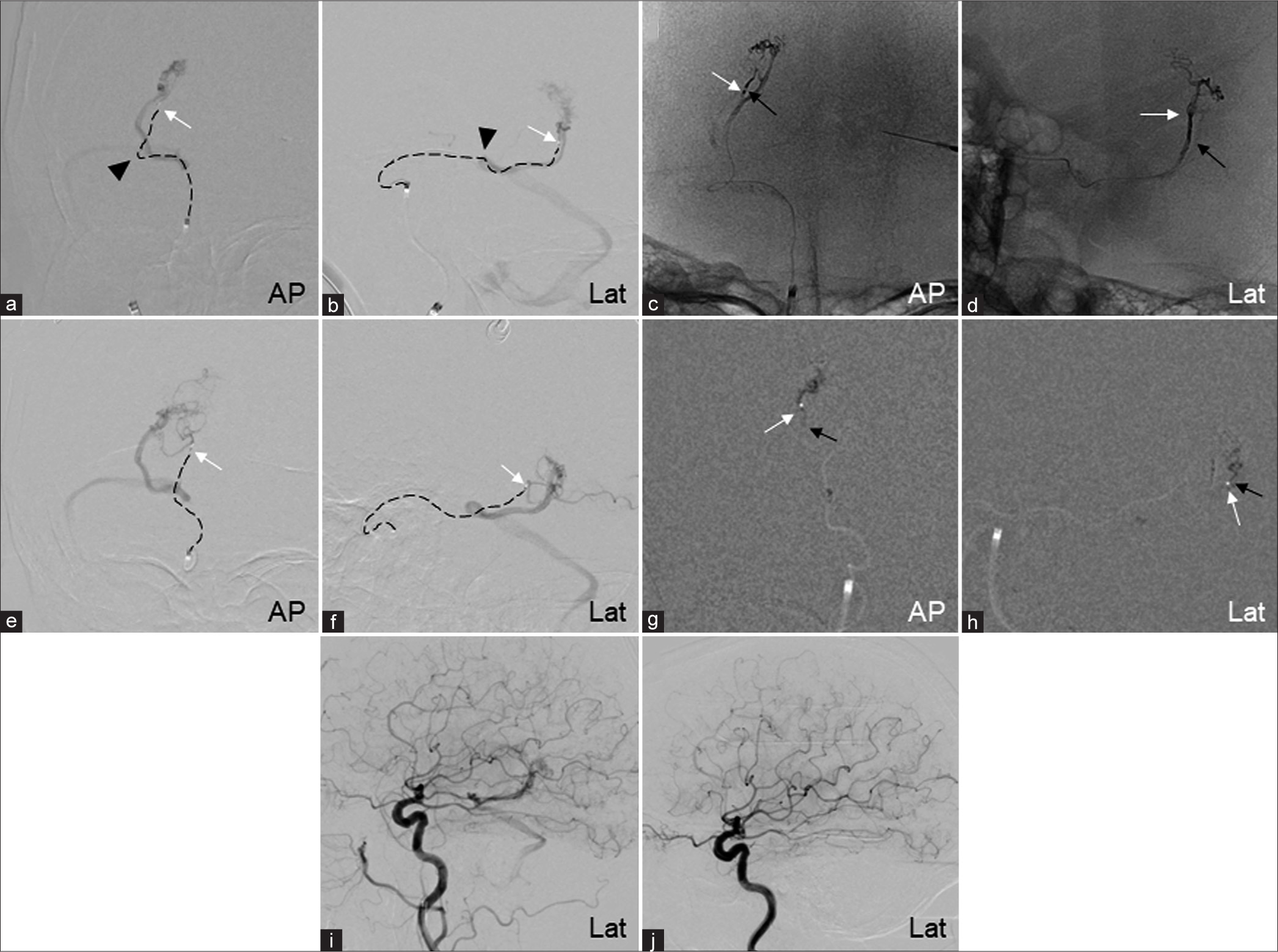
Figure 4:
Embolization of AVM through the right AChoA and LPChoA (Case 2) (a and b) Selective angiography of the AChoA. The microcatheter (dotted line) is advanced to the AChoA plexal segment. Black arrowheads show the choroidal point of the AChoA. Selective angiography shows AVM draining into the transverse-sigmoid sinus junction through inferior ventricular vein, basal vein of the rosenthal, and tentorial sinus. (c and d) Fluoroscopic images of the Onyx cast injected from the AChoA. (e and f) The microcatheter (dotted line) is advanced to the right posterior cerebral artery through the Pcom. Selective angiography shows the AVM fed by the LPChoA. (g and h) Fluoroscopic images of the NBCA cast injected from the LPChoA. (i) ICA angiography after embolization shows residual lesion with flow reduction. (j) The AVM disappeared on a follow-up angiography 3 years after gamma knife surgery (GKS). White and black arrows show the microcatheter’s tip and the reflux of the embolic agent, respectively. 3D-VR: Three-dimensional volume rendering, A-P: Anterior-posterior view, AChoA: Anterior choroidal artery, AVM: Arteriovenous malformation, ICA: Internal carotid artery, Lat: Lateral view, LPChoA: Lateral posterior choroidal artery, NBCA: n-butyl-2-cyanoacrylate, PChoA: Posterior choroidal artery, Pcom: Posterior communicating artery.
DISCUSSION
We performed successful embolization from the AChoA and PChoA for two ruptured ventricular AVMs without ischemic complications. Although ventricular AVMs within the ventricles are not eloquent locations, they are high-risk AVMs for treatment because they frequently possess deep drainers and require surgical removal through the eloquent cortex.[
There are high expectations for endovascular treatment in contributing to adjunctive or curative embolization. The main feeders of ventricular AVMs are AChoA, MPChoA, and LPChoA.[
Furthermore, it is important to understand the anatomical and angiographic features of PChoA for the safe embolization of ventricular AVMs.[
The LPChoA originates mainly from the P2 and P3 segments of the PCA and courses through the ambient cistern toward the choroidal fissure, between the thalamus and crus, the fornix, through the dorsal horn of the lateral ventricle to the body, and toward the foramen of Monro.[
From a literature review of AVMs with embolization through the PChoA, Ezura et al. reported the first safe embolization case of thalamic AVM through the PChoA as adjunctive embolization followed by GKS in 1992;[
We successfully embolized the ventricular AVMs from the AChoA and PChoA without complications by guiding the microcatheter to a more distal proper feeder. However, the safety of embolization may not be ensured by anatomical knowledge alone, even when embolization is performed beyond the angiographic safety point. Consequently, anatomical variations should be considered as unexpected complications can also occur. In addition, similar to the two cases reported here, the anastomosis of the AChoA and PChoA as complementary or that between the PChoA and the anterior cerebral artery should be noted,[
CONCLUSION
In this article, we reported the effectiveness of curative and adjunctive embolization of the choroidal arteries for ventricular AVMs. The microcatheter should be advanced as distally as possible to safely embolize the choroidal arteries while avoiding ischemic complications and the anatomical variation of the perforator branching position.
Declaration of patient consent
The Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
We would like to thank our radiological technologist for the technical assistance.
References
1. Bowden G, Kano H, Yang HC, Niranjan A, Flickinger J, Lunsford LD. Gamma Knife surgery for arteriovenous malformations within or adjacent to the ventricles. J Neurosurg. 2014. 121: 1416-23
2. Domingo RA, Grewal S, Tawk RG. Interhemispheric transcallosal approach for resection of choroidal arteriovenous malformation: Operative video. World Neurosurg. 2020. 136: 73
3. Dowd CF, Halbach VV, Barnwell SL, Higashida RT, Hieshima GB. Particulate embolization of the anterior choroidal artery in the treatment of cerebral arteriovenous malformations. AJNR Am J Neuroradiol. 1991. 12: 1055-61
4. Elkordy A, Endo H, Sato K, Matsumoto Y, Kondo R, Niizuma K. Embolization of the choroidal artery in the treatment of cerebral arteriovenous malformations. J Neurosurg. 2017. 126: 1114-22
5. Ezura M, Takahashi A, Yoshimoto T. Successful treatment of an arteriovenous malformation by chemical embolization with estrogen followed by conventional radiotherapy. Neurosurgery. 1992. 31: 1105-7 discussion 1107
6. Fernández-Miranda JC, De Oliveira E, Rubino PA, Wen HT, Rhoton AL. Microvascular anatomy of the medial temporal region: Part 1: Its application to arteriovenous malformation surgery. Neurosurgery. 2010. 67: ons237-76 discussion ons276
7. Fujii K, Lenkey C, Rhoton AL. Microsurgical anatomy of the choroidal arteries: Lateral and third ventricles. J Neurosurg. 1980. 52: 165-88
8. Hodes JE, Aymard A, Casasco A, Rüfenacht D, Reizine D, Merland JJ. Embolization of arteriovenous malformations of the temporal lobe via the anterior choroidal artery. AJNR Am J Neuroradiol. 1991. 12: 775-80
9. Hou K, Li C, Su H, Yu J. Imaging characteristics and endovascular treatment of brain arteriovenous malformations mainly fed by the posterior cerebral artery. Front Neurol. 2021. 11: 609461
10. Hou K, Li G, Luan T, Xu K, Yu J. The prospects and pitfalls in the endovascular treatment of moyamoya disease-associated intracranial aneurysms. Neurosurg Rev. 2021. 44: 261-71
11. Hou K, Xu K, Chen X, Ji T, Guo Y, Yu J. Targeted endovascular treatment for ruptured brain arteriovenous malformations. Neurosurg Rev. 2020. 43: 1509-18
12. Kim DJ, Kim DI, Lee SK, Kim SY. Homonymous hemianopia after embolization of an aneurysm-associated AVM supplied by the anterior choroidal artery. Yonsei Med J. 2003. 44: 1101-5
13. Ledezma CJ, Hoh BL, Carter BS, Pryor JC, Putman CM, Ogilvy CS. Complications of cerebral arteriovenous malformation embolization: Multivariate analysis of predictive factors. Neurosurgery. 2006. 58: 602-11
14. Lv X, Hu X, Li W, He H, Jiang C, Li Y. Curative and adjunctive AVM Onyx embolization of AVMs through the choroidal arteries. Interv Neuroradiol. 2017. 23: 392-8
15. Miyasaka Y, Yada K, Ohwada T, Morii S, Kitahara T, Kurata A. Choroid plexus arteriovenous malformations. Neurol Med Chir (Tokyo). 1992. 32: 201-6
16. Nagata S, Matsushima T, Fujii K, Takeshita I, Fukui M. Lateral ventricular arteriovenous malformations: Natural history and surgical indications. Acta Neurochir (Wien). 1991. 112: 37-46
17. Nataf F, Meder JF, Oppenheim C, Merienne L, Schlienger M. Radiosurgery of choroidal and cisternal cerebral arteriovenous malformations. Neurochirurgie. 2001. 47: 283-90
18. Neau JP, Bogousslavsky J. The syndrome of posterior choroidal artery territory infarction. Ann Neurol. 1996. 39: 779-88
19. Oran I, Parildar M, Derbent A. Ventricular/paraventricular small arteriovenous malformations: Role of embolisation with cyanoacrylate. Neuroradiology. 2005. 47: 287-94
20. Robert T, Cicciò G, Sylvestre P, Chiappini A, Weil AG, Smajda S. Anatomic and angiographic analyses of ophthalmic artery collaterals in moyamoya disease. AJNR Am J Neuroradiol. 2018. 39: 1121-6
21. Rothman SL, Allen WE, Simeone JF. The medial posterior choroidal artery as an indicator of masses at the foramen of Monro. Neuroradiology. 1976. 11: 123-9
22. Santoreneos S, Blumbergs PC, Jones NR. Choroid plexus arteriovenous malformations. A report of four pathologically proven cases and review of the literature. Br J Neurosurg. 1996. 10: 385-90
23. Slater LA, Hoffman C, Drake J, Krings T. Pre-operative embolization of a choroid plexus carcinoma: Review of the vascular anatomy. Childs Nerv Syst. 2016. 32: 541-5
24. Vinas FC, Lopez F. Dujovny M, Microsurgical anatomy of the posterior choroidal arteries. Neurol Res. 1995. 17: 334-44
25. Waga S. Surgical treatment of arteriovenous malformations in the lateral ventricle. Neurol Res. 1986. 8: 18-24
26. Walcott BP, Bang JS, Choudhri O, Gandhi S, Tabani H, Benet A. Contralateral transcallosal resection of a ventricular body arteriovenous malformation: 3D operative video. Neurosurg Focus. 2017. 43: V1
27. Yamauchi S, Kawakami T, Murata K, Ishiguro T, Ikeda H, Nishio A. Choroid plexus AVM with anomalous origin of the capsulothalamic artery: A case report. Interv Neuroradiol. 2018. 24: 76-81
28. Yu J, Xu N, Zhao Y, Yu J. Clinical importance of the anterior choroidal artery: A review of the literature. Int J Med Sci. 2018. 15: 368-75
29. Zeal AA, Rhoton AL. Microsurgical anatomy of the posterior cerebral artery. J Neurosurg. 1978. 48: 534-59