- Department of Neurosurgery, Moscow Regional Scientific Research Institute,
- Department of Neurosurgery, Russian Medical Academy for Continuing Professional Education, Moscow, Russia.
Correspondence Address:
Revaz Dzhindzhikhadze
Department of Neurosurgery, Russian Medical Academy for Continuing Professional Education, Moscow, Russia.
DOI:10.25259/SNI_727_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Revaz Dzhindzhikhadze1,2, Andrey Polyakov1, Oleg Dreval2, Valeriy Lazarev2. Successful microsurgical clipping of ruptured fusiform aneurysm of the anterior cerebral artery. Case report and review of the literature. 16-Dec-2020;11:445
How to cite this URL: Revaz Dzhindzhikhadze1,2, Andrey Polyakov1, Oleg Dreval2, Valeriy Lazarev2. Successful microsurgical clipping of ruptured fusiform aneurysm of the anterior cerebral artery. Case report and review of the literature. 16-Dec-2020;11:445. Available from: https://surgicalneurologyint.com/surgicalint-articles/10456/
Abstract
Background: Fusiform aneurysms (FA) of the anterior cerebral artery (ACA) are found rarely. The common clinical presentation is a subarachnoid hemorrhage (SAH). Surgery is the main treatment to prevent rebleeding.
Case Description: The authors present a case report of the ruptured FA of the ACA. The presented case demonstrates the successful microsurgical clipping of the fusiform ACA aneurysm.
Conclusion: A1-segment FA can lead to SAH with poor prognosis. The main goal of surgical treatment is to prevent rebleeding. Direct microsurgical clipping is one of the surgical options.
Keywords: Anterior cerebral artery, Fusiform aneurysm, Subarachnoid hemorrhage
INTRODUCTION
Aneurysms of the proximal anterior cerebral artery (ACA) are quite rare, accounting for 0.7– 1.4% of all intracranial aneurysms and 3–4% of ACA aneurysms.[
Dissecting FA of the anterior circulation are one of the causes of ischemic stroke and subarachnoid hemorrhage (SAH) among the young patients.[
We present a case report – successful microsurgical clipping of a ruptured dissecting FA in the A1-segment of ACA.
CASE PRESENTATION
Patient, 45-years-old female, was admitted in hospital on the next day after intracranial hemorrhage.
It is known that the onset of the disease was with a sudden headache, pain in cervico-occipital localization and rise of the arterial blood pressure up to 220 mm Hg. On admission to hospital a condition was critical, 11 Glasgow Coma Scale, Hunt-Hess 3 meningeal signs. The pupils are equal. Oculomotor dysfunction and visible paresis are not detected. The Babinsky signs on both sides.
Data on the previous infections, craniocerebral injuries and systemic connective tissue disorders are not detected.
Transcranial Doppler verified the rise of cerebral blood flow up to 220 cm/s.
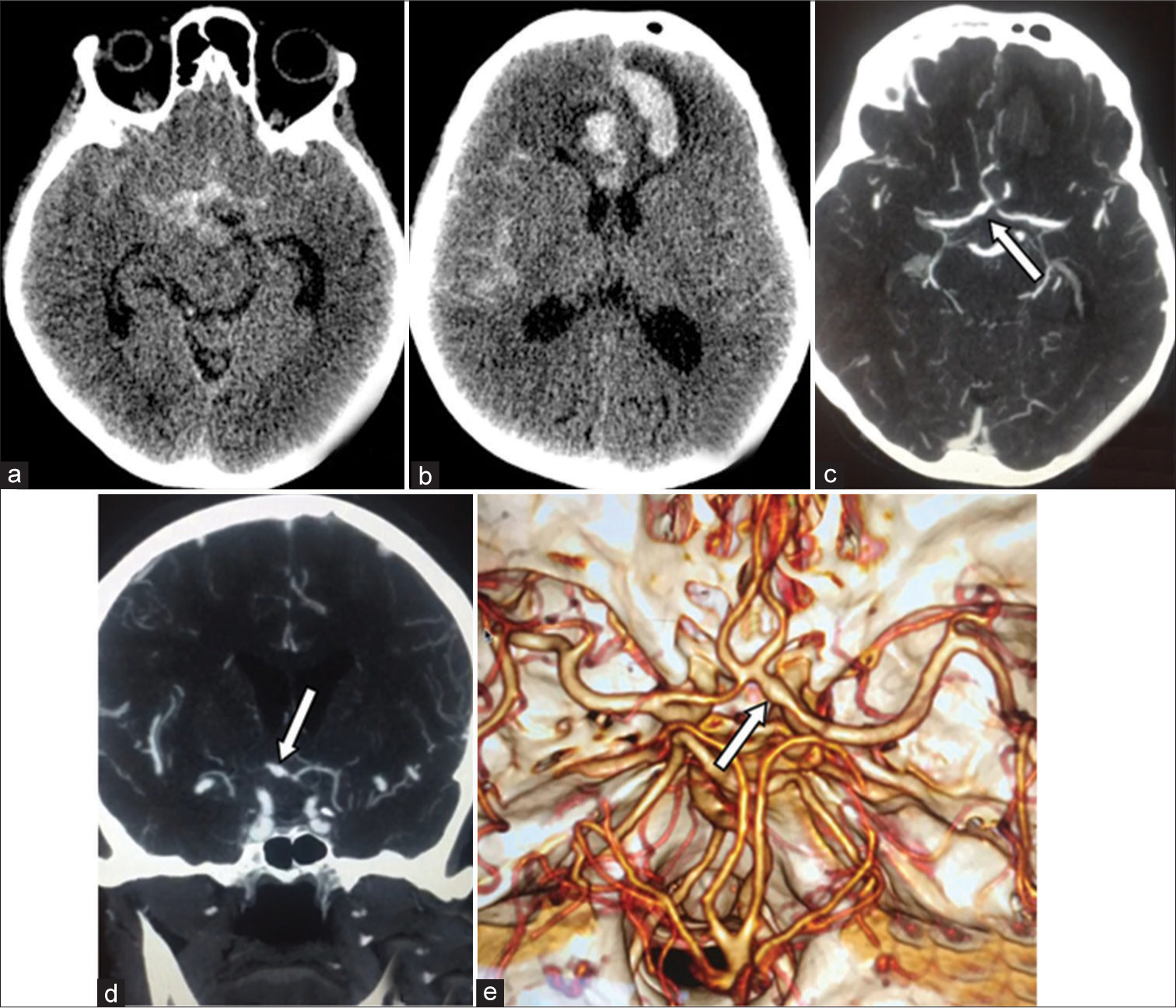
Brain computed tomography (CT) scan and CT-angiography revealed the right sided FA on the A1-segment of ACA, basal SAH, intracerebral hematoma in the mediobasal frontal lobe and in the interhemispheric fissure [
Figure 1:
(a and b) Native computed tomography (CT) scan. Subarachnoid-parenchymal hemorrhage associated with development of the intracerebral hematoma in the interhemispheric fissure and the left cortex. (c-e) CT-angiography. In the area of the A1-segment of the right anterior cerebral artery – is an extended fusiform aneurysm in the distal A1-segments (arrow); 6.6 mm in diameter at widest part of fusiform aneurysm and a length of about 10.4 mm.
Because of a critical condition of the patient, we decided to treat the patient conservatively. The main protocol includes treatment of cerebral vasospasm and intracranial hypertension. This choice was explained by the possible need for trapping the A1-segment with revascularization, which could lead to worsen of the patient’s condition and an unfavorable outcome.
After 12 days, when the patient condition was stabilized we performed a right-sided lateral supraorbital craniotomy, microsurgical clipping of the eccentrically FA on the A1 segment of ACA.
Traditional microneurosurgical technique was used. The aneurysmal dilatation occupied the distal part of the A1 segment. Along with fibrin masses in the area of the rupture the eccentrically fusiformed part of the aneurysm was visualized which was adhered to the optic nerve. In this case, the posterior wall of the artery was not changed. Aneurysm was dissected and clipped. There were no intraoperative complications.
The patient was extubated in the operating room and transferred to the intensive care unit. The postoperative period was uneventful. On the CT scan there are no postoperative complications.
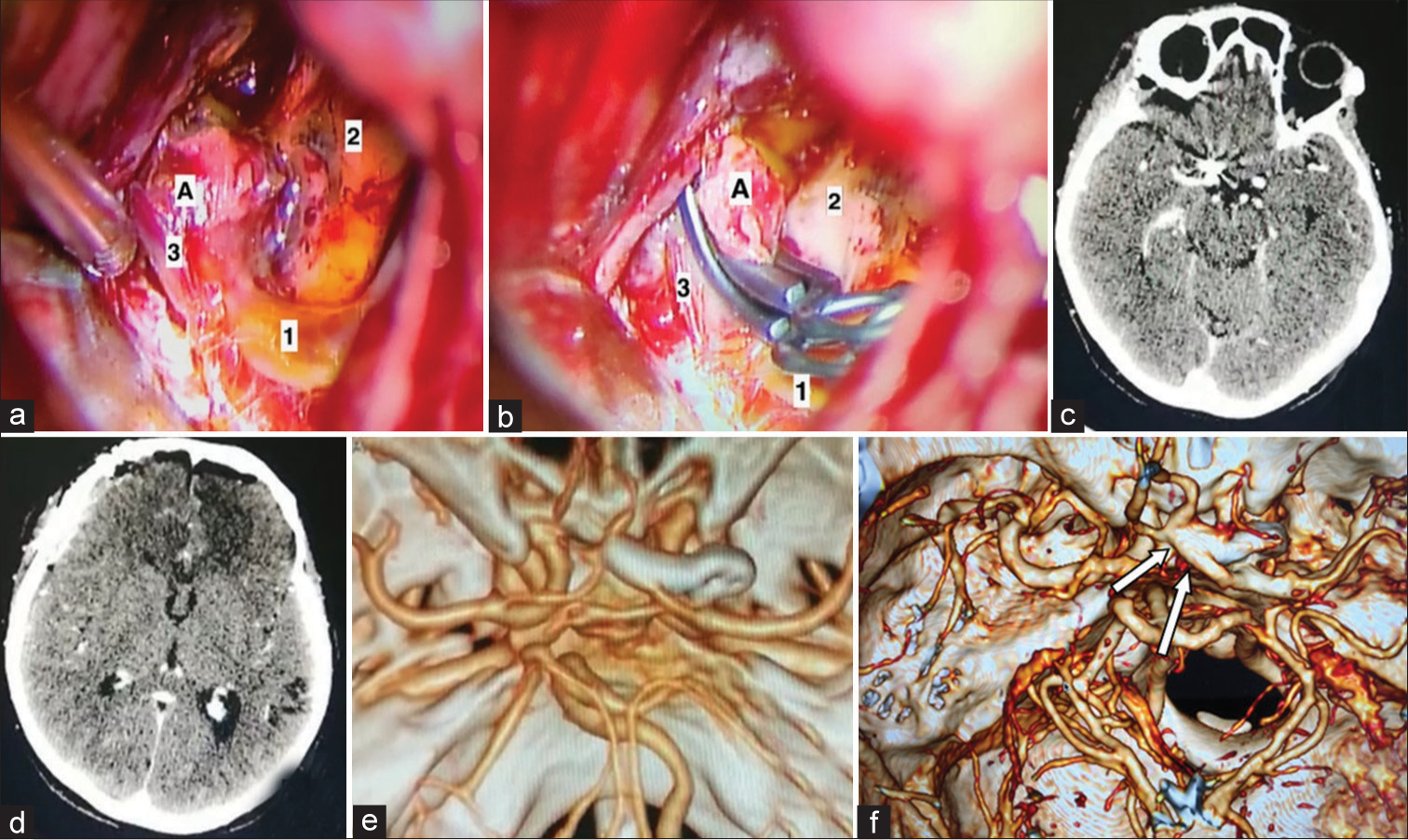
Intraoperative photo and the CT scans of the brain in [
Figure 2:
(a and b) A microsurgical clipping stages of the eccentrically fusiformed part of the aneurysm of the A1-segment of the right part of the anterior cerebral artery (ACA), (1) A1-segment of the ACA, (2) the right optic nerve, (3) the recurrent artery of Heubner, A - aneurysm. (c and d) Postoperative changes on native computed tomography (CT)-scan. (e and f) CT-angiography shows that the aneurysm was clipped. The ipsilateral A1 segment of the right ACA is conserved (arrows).
The patient was discharged without neurological deficit on the day 9th day after surgery.
DISCUSSION
FA of the proximal ACA is a rare pathology. Only few cases are described in the literature.[
Surgical technics are controversial and depend on the size of the aneurysm and individual anatomy of circle of Willis. Clipping is the method of choice; however, the traditional technique is quite complicated due to the fusiform shape and high risk of intraoperative rupture. Clipping with a fenestrated clip can be used for affected segment; but the risk of damaging the perforating arteries and the recurrent artery of Heubner are high.[
SAH due to ruptured dissecting aneurysm of anterior circulation is an infrequent pathology.[
Mizutani et al. found that the destruction of the inner elastic membrane of the artery is the trigger of the development of dissection. At the time of damage of the membrane blood enters the vessel wall, forming a pseudo-lumen which causes an arterial stenosis. Thereafter, the blood flow can fall back into the true lumen, or into the layer between adventitia and media, which subsequently can cause SAH. The study also described that the vessel wall begins to restore from the 1st week of dissection and the reparative process lasts for about 4–5 weeks.[
It is not always possible to verify a dissecting aneurysm only according to cerebral angiography. One of the main signs is a double lumen, stenosis, and dilatation (“a pearl and a string symptom”), isolated stenosis (“a string symptom”), or occlusion.[
Over the past two decades, there have been many reports of dissecting FA and SAH, which may be due to their inadequate diagnosis. For example, hypoplasia of the A1 segment can be regarded as dissection and such conclusion can occur with a frequency of up to 29%.[
The main goal of the treatment is to prevent hemorrhage, which can be achieved by both open surgery and endovascular obliteration. In this example, the treatment consisted of microsurgical clipping. However, there are cases when direct clipping was insufficient and led to repeated hemorrhage.[
One of the treatment options is to use flow-diverting stents, but this is also not a safe procedure.[
This clinical case demonstrates the importance of choosing the right treatment strategy in the form of determining the timing and type of surgery. Severe initial condition of the patient with massive subarachnoid-parenchymal hemorrhage on CT could lead to an unfavorable outcome. Hence, the strategy of surgical intervention in the delayed post rupture stage was chosen, which led to a favorable outcome. To determine the optimal strategy, a larger number of observations are needed with evaluation of follow-up data.
CONCLUSION
Ruptured fusiform dissecting aneurysms of proximal ACA can cause severe intracranial hemorrhage and devastating consequences. The purpose of surgical treatment is to prevent repeated hemorrhage. In presented case, microsurgical clipping provided obliteration of the aneurysm and successful outcome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aoki Y, Nemoto M, Yokota K, Kano T, Goto S, Sugo N. Ruptured fusiform aneurysm of the proximal anterior cerebral artery (A1 segment). Neurol Med Chir (Tokyo). 2007. 47: 351-5
2. Dashti R, Hernesniemi J, Lehto H, Niemelä M, Lehecka M, Rinne J. Microneurosurgical management of proximal anterior cerebral artery aneurysms. Surg Neurol. 2007. 68: 366-77
3. De Jesús O. Giant aneurysm of the proximal anterior cerebral artery. Surg Neurol. 1996. 46: 553-6
4. Evans AL, Corkill RA, Wenderoth JD. Ruptured fusiform aneurysm of fenestrated A1 segment of the anterior cerebral artery. Case report and review of the literature. Neuroradiology. 2006. 48: 196-9
5. Hatayama K, Karasawa H, Naito H, Hirota N, Sugiyama K, Ueno J. Anterior cerebral artery dissecting aneurysm associated with fibromuscular dysplasia (FMD): A case report. No Shinkei Geka. 2001. 29: 451-6
6. Hino A, Fujimoto M, Iwamoto Y, Oka H, Echigo T. Surgery of proximal anterior cerebral artery aneurysms. Acta Neurochir (Wien). 2002. 144: 1291-6
7. Im TS, Lee YS, Suh SJ, Lee JH, Ryu KY, Kang DG. Two cases of subarachnoid hemorrhage from spontaneous anterior cerebral artery dissection: A case of simultaneous hemorrhage and ischemia without aneurysmal formation and another case of hemorrhage with aneurysmal formation. J Cerebrovasc Endovasc Neurosurg. 2014. 16: 119-24
8. Inoue T, Fujimura M, Matsumoto Y, Kondo R, Inoue T, Shimizu H. Simultaneous occurrence of subarachnoid hemorrhage and cerebral infarction caused by anterior cerebral artery dissection treated by endovascular trapping. Neurol Med Chir. 2010. 50: 574-7
9. Jeliava S.editors. Hirurgicheskoe Lechenie Anevrizm Golovnogo Mozga v Ostrom Periode Krovoizlijanija. Moscow: T.A. Alekseeva; 2019. p.
10. Kashimura H, Mase T, Ogasawara K, Ogawa A, Endo H. Trapping and vascular reconstruction for ruptured fusiform aneurysm in the proximal A1 segment of the anterior cerebral artery. Neurol Med Chir (Tokyo). 2006. 46: 340-3
11. Krylov V.editors. Mikrohirurgija Anevrizm Villizieva Mnogougol’nika. Moscow: American Heart Association; 2004. p.
12. Kunze S, Schiefer W. Angiographic demonstration of a dissecting aneurysm of the middle cerebral artery. Neuroradiology. 1971. 2: 201-6
13. Kurino M, Yoshioka S, Ushio Y. Spontaneous dissecting aneurysms of anterior and middle cerebral artery associated with brain infarction: A case report and review of the literature. Surg Neurol. 2002. 57: 428-38
14. Mizutani T, Kojima H, Asamoto S, Miki Y. Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurg. 2001. 94: 712-7
15. Nagahata M, Seino H, Kakehata S, Morimoto K, Nakano T, Asano K. Dilated outer diameter of the dissected artery: Acute bilateral anterior cerebral artery dissection evaluated by repeat magnetic resonance cisternography. Case report. Neurol Med Chir (Tokyo). 2010. 50: 1095-8
16. Nomura M, Kida S, Kita D, Higashi R, Hasegawa M, Matsui O. Fusiform aneurysm of the proximal anterior cerebral artery (A1). Acta Neurochir (Wien). 2000. 142: 1163-4
17. Oba M, Suzuki M, Onuma T. Two cases of ruptured fusiform aneurysm of the proximal anterior cerebral artery (A1 segment). No Shinkei Geka. 1989. 17: 365-8
18. Ohkuma H, Suzuki S, Kikkawa T, Shimamura N. Neuroradiologic and clinical features of arterial dissection of the anterior cerebral artery. AJNR Am J Neuroradiol. 2003. 24: 691-9
19. Ohkuma H, Suzuki S, Ogane K. Dissecting aneurysms of intracranial carotid circulation. Stroke. 2002. 33: 941-7
20. Sakima H, Isa K, Goya Y, Ohya Y. Sagittal MR black blood imaging revealing ACA dissection. Intern Med. 2012. 51: 1145
21. Shigemori M, Kawaba T, Yoshitake Y, Moritaka K, Miyagi J, Kuramoto S. Fusiform aneurysm of the proximal (A1 portion) anterior cerebral artery. Surg Cerebral Stroke. 1987. 15: 51-5
22. Stebens W, Fox JL.editors. The pathology of intracranial arterial aneurysms and their complications. Intracranial Aneurysms. New York, Berlin, Heidelberg, Tokyo: Springer-Verlag; 1983. 1: 272-357
23. Suzuki K, Mishina M, Okubo S, Abe A, Suda S, Ueda M. Anterior cerebral artery dissection presenting subarachnoid hemorrhage and cerebral infarction. J Nippon Med Sch. 2012. 79: 153-8
24. Suzuki M, Onuma T, Sakurai Y, Mizoi K, Ogawa A, Yoshimoto T. Aneurysms arising from the proximal (A1) segment of the anterior cerebral artery. A study of 38 cases. J Neurosurg. 1992. 76: 455-8
25. Suzuki M, Onuma T, Sakurai Y, Suzuki J. Twenty-six cases regarding the proximal anterior cerebral artery. No Shinkei Geka. 1988. 16: 701-5
26. Tamura M, Tsukahara Y, Yodonawa M. Fusiform aneurysm of the anterior cerebral artery (A1 segment)--a case report. No Shinkei Geka. 1985. 13: 1337-40
27. Tanikawa R, Anei R, Izumi N, Hashizume A, Fujita T, Hashimoto M. Strategy of treatment for anterior cerebral artery dissection. Surg Cerebral Stroke. 1999. 27: 433-8
28. Tekkök IH, Açikgöz B. Giant aneurysm of the proximal (A1) anterior cerebral artery. Acta Neurochir (Wien). 2001. 143: 1287-92
29. Thines L, Zairi F, Taschner C, Leclerc X, Lucas C, Bourgeois P. Subarachnoid hemorrhage from spontaneous dissection of the anterior cerebral artery. Cerebrovasc Dis (Basel Switzerland). 2006. 22: 452-6
30. Uozumi Y, Katoh H, Tsuzuki N, Toyooka T, Miyazawa T, Nawashiro H. Revascularization for anterior cerebral artery dissecting aneurysms--three case reports. Neurol Med Chir. 2010. 50: 49-53
31. van Rooij WJ, Sluzewski M. Perforator infarction after placement of a pipeline flow-diverting stent for an unruptured A1 aneurysm. AJNR Am J Neuroradiol. 2010. 31: E43-4
32. Velthuis BK, van Leeuwen MS, Witkamp TD, Ramos LM, van der Sprenkel JW, Rinkel GJ. Surgical anatomy of the cerebral arteries in patients with subarachnoid hemorrhage: Comparison of computerized tomography angiography and digital subtraction angiography. J Neurosurg. 2001. 95: 206-12
33. Wakabayashi T, Tamaki N, Yamashita H, Saya H, Suyama T, Matsumoto S. Angiographic classification of aneurysms of the horizontal segment of the anterior cerebral artery. Surg Neurol. 1985. 24: 31-4
34. Wanibuchi M, Kurokawa Y, Ishiguro M, Fujishige M, Inaba K. Characteristics of aneurysms arising from the horizontal portion of the anterior cerebral artery. Surg Neurol. 2001. 55: 148-55
35. Yasargil MG, Smith RD, Young PH, Teddy PJ.editors. Anterior cerebral and anterior communicating arteria aneurysms. Microneurosurgery II. Clinical Considerations, Surgery of the Intracranial Aneurysms and Results. Stuttgart: Georg-Thieme-Verlag; 1984. p. 165-231
36. Yonas H, Agamanolis D, Takaoka Y, White RJ. Dissecting intracranial aneurysms. Surg Neurol. 1977. 8: 407-15