- Department of Neurosurgery, Faculty of Medicine, The University of Tokyo, Tokyo, Japan
- Department of Orthopaedic Surgery, Faculty of Medicine, The University of Tokyo, Tokyo, Japan
Correspondence Address:
Hideaki Imai
Department of Neurosurgery, Faculty of Medicine, The University of Tokyo, Tokyo, Japan
DOI:10.4103/2152-7806.191072
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Imai H, Ishii K, Chikuda H, Ohya J, Nakagawa D, Kondo T, Nomura S, Yoshino M, Miyawaki S, Kin T, Nakatomi H, Saito N. Successful surgical strategy for a cervical hemangioblastoma: Case report. Surg Neurol Int 22-Sep-2016;7:
How to cite this URL: Imai H, Ishii K, Chikuda H, Ohya J, Nakagawa D, Kondo T, Nomura S, Yoshino M, Miyawaki S, Kin T, Nakatomi H, Saito N. Successful surgical strategy for a cervical hemangioblastoma: Case report. Surg Neurol Int 22-Sep-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/successful-surgical-strategy-cervical-hemangioblastoma-case-report/
Abstract
Background:Hemangioblastomas are hypervascular lesions and hence their surgical management is challenging. In particular, if complete resection is to be attained, all feeding and draining vessels must be occluded. Although most intramedullary spinal cord tumors are treated utilizing a posterior approach, we describe an anterior surgical strategy for resection of an intramedullary cervical hemangioblastoma.
Case Description:A 36-year-old female with a spinal hemangioblastoma located in the anterior cervical spinal cord presented with a long-standing history of motor weakness of the right upper extremity. Magnetic resonance imaging revealed a large multilevel extensive syrinx and a focal intramedullary enhanced tumor at the C6 level. Angiography showed that the main feeder to the tumor was the left radicular artery (C8), which originated from the thyrocervical trunk, penetrated the dura mater, and branched both rostrally and caudally into the anterior spinal artery (ASA). Three-dimensional computer graphic images showed the tumor was located in the anterior part of the spinal cord, adjacent to and supplied by the ASA. The planned anterior surgical approach involved a total corpectomy of C6 and partial corpectomies of C5 and C7. The tumor was entirely removed despite multiple adhesions, and was successfully freed from the ASA. Patency of the ASA was confirmed utilizing intraoperative indocyanine green videoangiography. Intraoperatively, no monitoring changes were encountered. The pathological diagnosis was of a hemangioblastoma. No postoperative deficit occurred.
Conclusions:An anterior approach for the resection of an anteriorly located intramedullary spinal hemangioblastomas was successfully accomplished in this case.
Keywords: Anterior approach, hemangioblastoma, indocyanine green, spinal cord, temporary arterial occlusion, three-dimensional computer graphics
INTRODUCTION
Spinal hemangioblastoma is the third most common intrinsic intramedullary tumor, and accounts for approximately 5% of all spinal cord tumors.[
CASE DESCRIPTION
A 36-year-old female presented with weakness of her right upper extremity of 10 years duration, accompanied by progressive muscle atrophy of the right hand. Recently, she had started experiencing increasing neck pain and numbness in both her upper and lower extremities. Magnetic resonance (MR) imaging of the cervical spine demonstrated an anterolateral enhanced lesion opposite the C6 vertebral body accompanied by a multilevel proximal/distal syrinx extending from the medulla to the T4 level [Figures
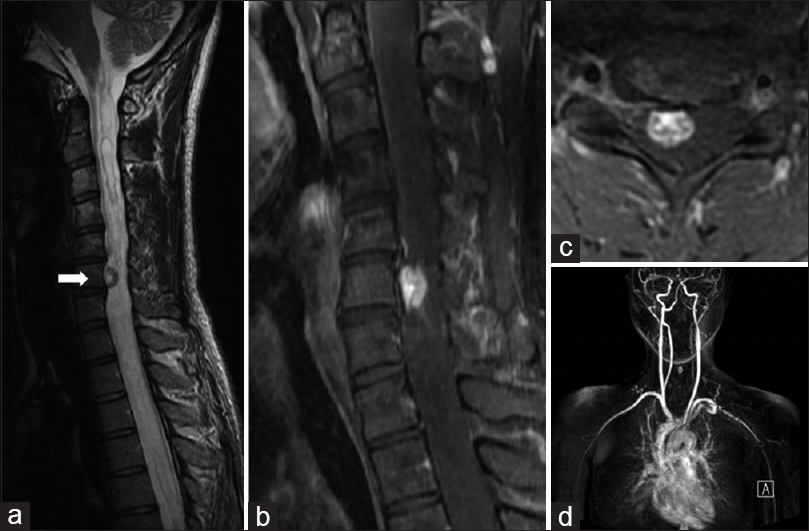
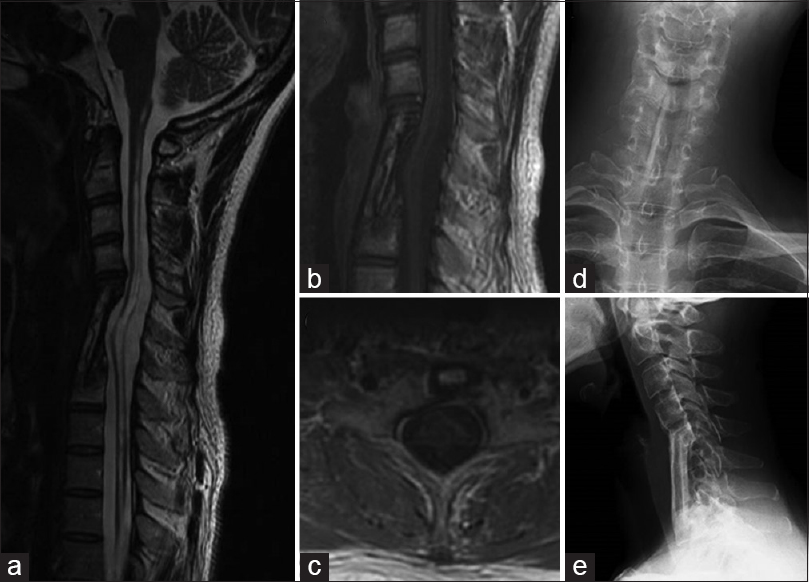
Figure 1
(a) Preoperative sagittal T2-weighted magnetic resonance (MR) image showing extensive syringobulbia and syringomyelia and a small mass (arrow) in the spinal cord at C6. (b and c) Preoperative sagittal and axial gadolinium (Gd)-enhanced MR images of the cervical spine showing a small enhanced mass in the spinal cord at C6 (b) and in the right anterior quadrant of the spinal cord (c). (d) Gd-enhanced MR angiogram of the upper parts of the body showing tumor staining in the cervical spine
MR angiography with gadolinium-diethylenet riaminepenta-acetic acid of the upper body demonstrated the C6 intramedullary anterolateral spinal cord tumor, but no other lesions [
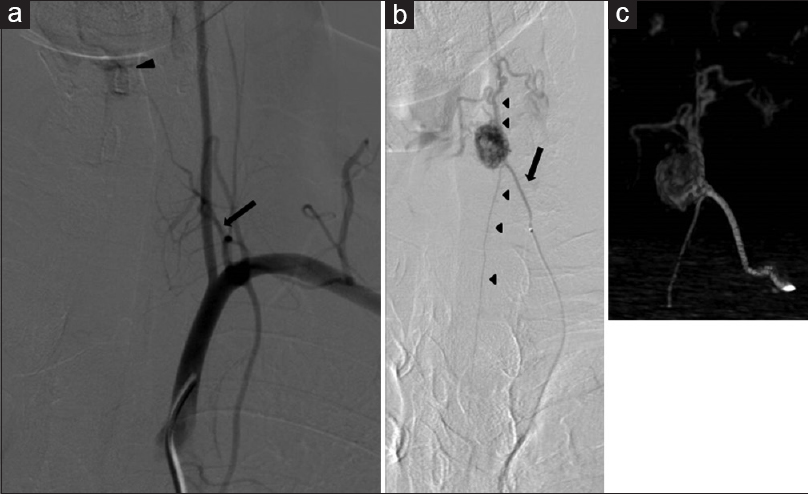
Figure 2
(a) Left subclavian artery angiogram showing the thyrocervical artery (arrow) as a feeder of the tumor (arrowhead). (b) Selective angiogram of the thyrocervical trunk showing the radicular artery entering (arrow) the spinal canal with the C8 root, branching of the anterior spinal artery (rostral and caudal) (arrowhead) and the feeding artery at C6, tumor staining, and the draining vein. (c) Three-dimensional rotational selective angiogram of the thyrocervical trunk showing the tumor location and surrounding vessels
3D computed tomography (CT) angiography confirmed the same findings as the MR imaging with gadolinium [
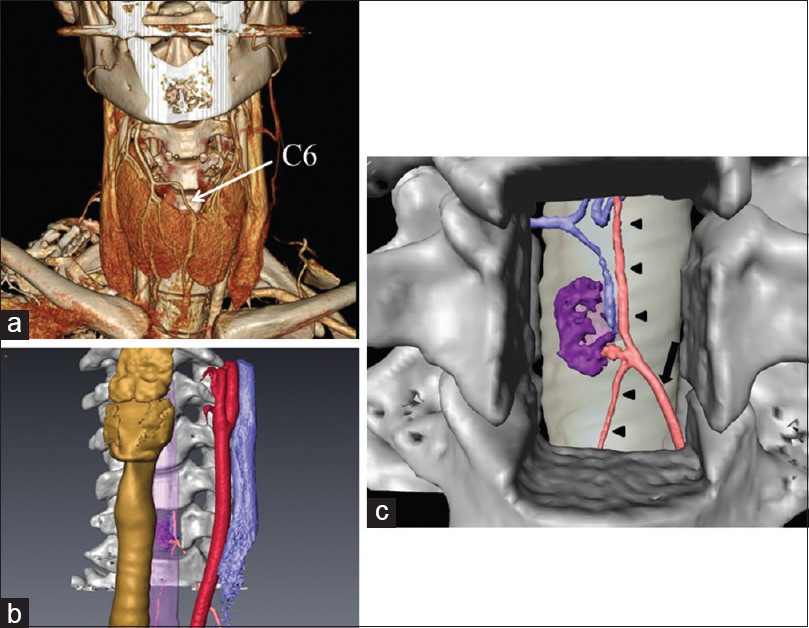
Figure 3
(a) Three-dimensional (3D) computed tomography angiogram showing C6 and surrounding structures. (b) 3D computer graphic image showing the anatomical relationships of the spinal tumor (purple) and feeding artery (pink), and the cervical vertebral bodies (white), trachea (yellow), esophagus (purple), common carotid artery (red), and jugular vein (blue). (c) Planned corpectomy of C6 and simulated surgical view showing the anterior radicular artery (pink and arrow), anterior spinal artery (pink and arrowheads), tumor (purple), and drainer (blue)
Surgical resection
The intramedullary tumor was removed via an anterior approach utilizing monitoring of motor evoked potentials (MEPs) and somatosensory evoked potentials (SSEPs).[
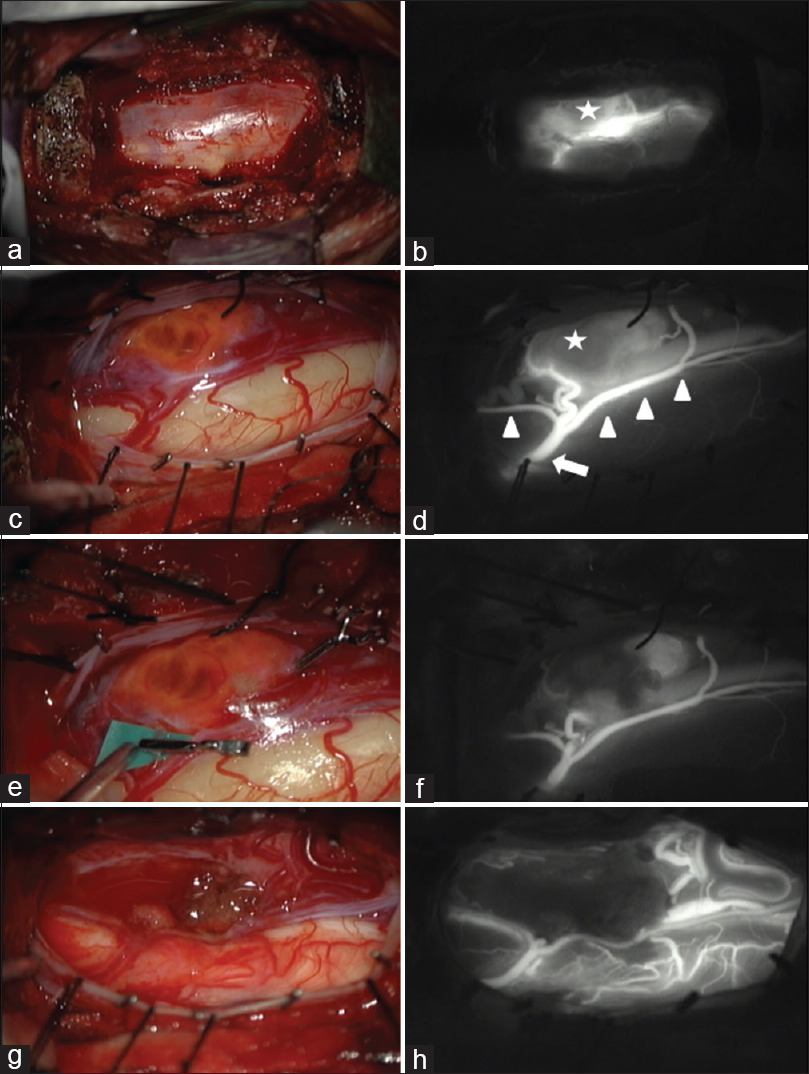
Figure 4
Surgical microscopic images. (a) After total corpectomy of C6 and partial corpectomies of C5 and C7. (b) Indocyanine green (ICG) injection showing the tumor stain (star), the anterior spinal artery, and surrounding venous drainage. (c) Opening of the dura mater exposed these structures. (d) ICG injection clearly showing the anterior radicular artery (arrow), anterior spinal artery (arrowheads), and the tumor (star). (e) Temporary clip was applied to the feeding artery of the tumor. (f) ICG injection showing reduced blood supply. (g) Dissection of the tumor. (h) ICG injection showing no residual tumor and the intact anterior spinal cord artery
Postoperatively, the patient remained neurologically unchanged with similar motor function, however, had slight improvement in upper extremity numbness. Her dysphagia was only temporary, resolving over the 1st postoperative month. Postoperative MR imaging confirmed total removal of the tumor and shrinkage of the associated syrinx. The pathological diagnosis was hemangioblastoma. The halo vest external fixation was removed 2 months after the surgery and replaced with a neck collar until confirmation of adequate bone fusion. Follow-up MR imaging at 1.5 years confirmed total removal of the tumor and shrinkage of the associated syrinx with accompanying atrophy of the spinal cord [Figures
Figure 5
Follow-up MR images 1.5 years after the surgery. (a) Sagittal T2-weighted MR image showing disappearance of the tumor and collapse of the syrinx. (b and c) Sagittal (b) and axial (c) Gd-enhanced MR images revealing total removal of the tumor. (d and e) Postoperative cervical radiographs, anteroposterior (d) and lateral (e) views, showing good graft bone fusion
DISCUSSION
The standard surgical approach to intramedullary spinal cord tumor is posterior and typically involves laminectomy or laminoplasty.[
In the present case, meticulous microsurgical dissection of proximal portions of the feeding artery allowed temporary arterial occlusion with a Sugita AVM Microclip at the tumor edge [Figure
CONCLUSION
This case study demonstrates how anterior resection (e.g., C5-C7 partial/complete corpectomy/plating) of an anterolateral intramedullary C6 hemangioblastoma may be successfully achieved. Planning based on presurgical simulation with 3D computer graphic images and intraoperative ICG videoangiography with temporary artery occlusion under neuromonitoring helped achieve safe gross total excision of this lesion with preservation of the ASA.
Financial support and sponsorship
This work was supported by a Grant-in-Aid for Scientific Research (C) (No. 25462206) to H.I. from the Japan Society for the Promotion of Science.
Conflicts of interest
The authors report no conflicts of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
1. Baleriaux DL. Spinal cord tumors. Eur Radiol. 1999. 9: 1252-8
2. Brotchi J. Intrinsic spinal cord tumor resection. Neurosurgery. 2002. 50: 1059-63
3. Clark AJ, Lu DC, Richardson RM, Tihan T, Parsa AT, Chou D. Surgical technique of temporary arterial occlusion in the operative management of spinal hemangioblastomas. World Neurosurg. 2010. 74: 200-5
4. Hwang SW, Malek AM, Schapiro R, Wu JK. Intraoperative use of indocyanine green fluorescence videography for resection of a spinal cord hemangioblastoma. Neurosurgery. 2010. 67: ons300-3
5. Iwasaki Y, Koyanagi I, Hida K, Abe H. Anterior approach to intramedullary hemangioblastoma: Case report. Neurosurgery. 1999. 44: 655-7
6. Martin NA, Khanna RK, Batzdorf U. Posterolateral cervical or thoracic approach with spinal cord rotation for vascular malformations or tumors of the ventrolateral spinal cord. J Neurosurg. 1995. 83: 254-61
7. Oppenlander ME, Spetzler RF. Advances in spinal hemangioblastoma surgery. World Neurosurg. 2010. 74: 116-7
8. Pluta RM, Wait SD, Butman JA, Leppig KA, Vortmeyer AO, Oldfield EH. Sacral hemangioblastoma in a patient with von Hippel-Lindau disease. Case report and review of the literature. Neurosurg Focus. 2003. 15: E11-
9. Watanabe K, Watanabe T, Takahashi A, Saito N, Hirato M, Sasaki T. Transcranial electrical stimulation through screw electrodes for intraoperative monitoring of motor evoked potentials. Technical note. J Neurosurg. 2004. 100: 155-60