- Department of Neurosurgery, Ube-kohsan Central Hospital, 750 Nishikiwa, Ube, Yamaguchi, Japan
Correspondence Address:
Makoto Ideguchi
Department of Neurosurgery, Ube-kohsan Central Hospital, 750 Nishikiwa, Ube, Yamaguchi, Japan
DOI:10.4103/sni.sni_317_18
Copyright: © 2019 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Natsumi Fujii, Makoto Ideguchi, Takafumi Nishizaki, Norio Ikeda, Taichi Shimabukuro, Tomomi Okamura, Machiko Oono. Successful treatment of a case of tentorial dural arteriovenous fistula causing subarachnoid hemorrhage with invagination of the brainstem by huge and multiple venous pouches. 15-Jan-2019;10:2
How to cite this URL: Natsumi Fujii, Makoto Ideguchi, Takafumi Nishizaki, Norio Ikeda, Taichi Shimabukuro, Tomomi Okamura, Machiko Oono. Successful treatment of a case of tentorial dural arteriovenous fistula causing subarachnoid hemorrhage with invagination of the brainstem by huge and multiple venous pouches. 15-Jan-2019;10:2. Available from: http://surgicalneurologyint.com/surgicalint-articles/9168/
Abstract
Background:We present a case of tentorial dural arteriovenous fistula (TDAVF) causing subarachnoid hemorrhage with mass effect of large venous pouches, which was struggling to diagnosis and management due to complex vasculature and severe general condition.
Case Description:A 43-year-old man was transferred to our hospital due to sudden consciousness disturbance. A neurological examination revealed tetraparesis and pupil dilatation with no light reflex. Imaging findings showed a large lesion in the brainstem with subarachnoid and intraventricular hemorrhage. Since there were multiple feeding arteries and large and multiple venous pouches on vascular imaging, we diagnosed the patient with TDAVF. Because of a high-flow arteriovenous shunt and the presence of large venous pouches, it appeared to be very difficult to approach the shunting point by direct surgery. Therefore, we first performed transarterial endovascular treatment with 25% n-butyl-2-cyanoacrylate to shrink the venous pouches and to reduce the pressure of the posterior fossa, followed by direct radical interruption of the shunting point using the craniotomy maneuver. Postoperative vascular imaging revealed disappearance of abnormal feeding arteries, draining veins, and venous pouches. The patient was discharged and transferred to a rehabilitation hospital with a modified Rankin Scale Score of 3. Accurate interpretation of the detailed vasculature preoperatively and an appropriate treatment strategy using endovascular and direct surgical technique are required to achieve a satisfactory outcome for difficult-to-treat dural arteriovenous fistulas.
Conclusions:This combined maneuver with endovascular embolism as complementary pretreatment for radical surgery is useful for a case with high-flow shunting and large venous pouches.
Keywords: Combined treatment with endovascular and direct surgery, multiple venous pouches, subarachnoid hemorrhage, tentorial dural arteriovenous fistula
INTRODUCTION
Dural arteriovenous fistula (DAVF) accounts for 10–15% of all intracranial vascular malformations.[
CASE HISTORY
A 43-year-old man was transferred to our hospital due to sudden loss of consciousness. Neurological examination revealed tetraparesis and pupil dilatation with no light reflex. The Glasgow Coma Scale Score was 4 (E1, V1, M2). His condition immediately deteriorated to ataxic respiration, and he then underwent intratracheal intubation.
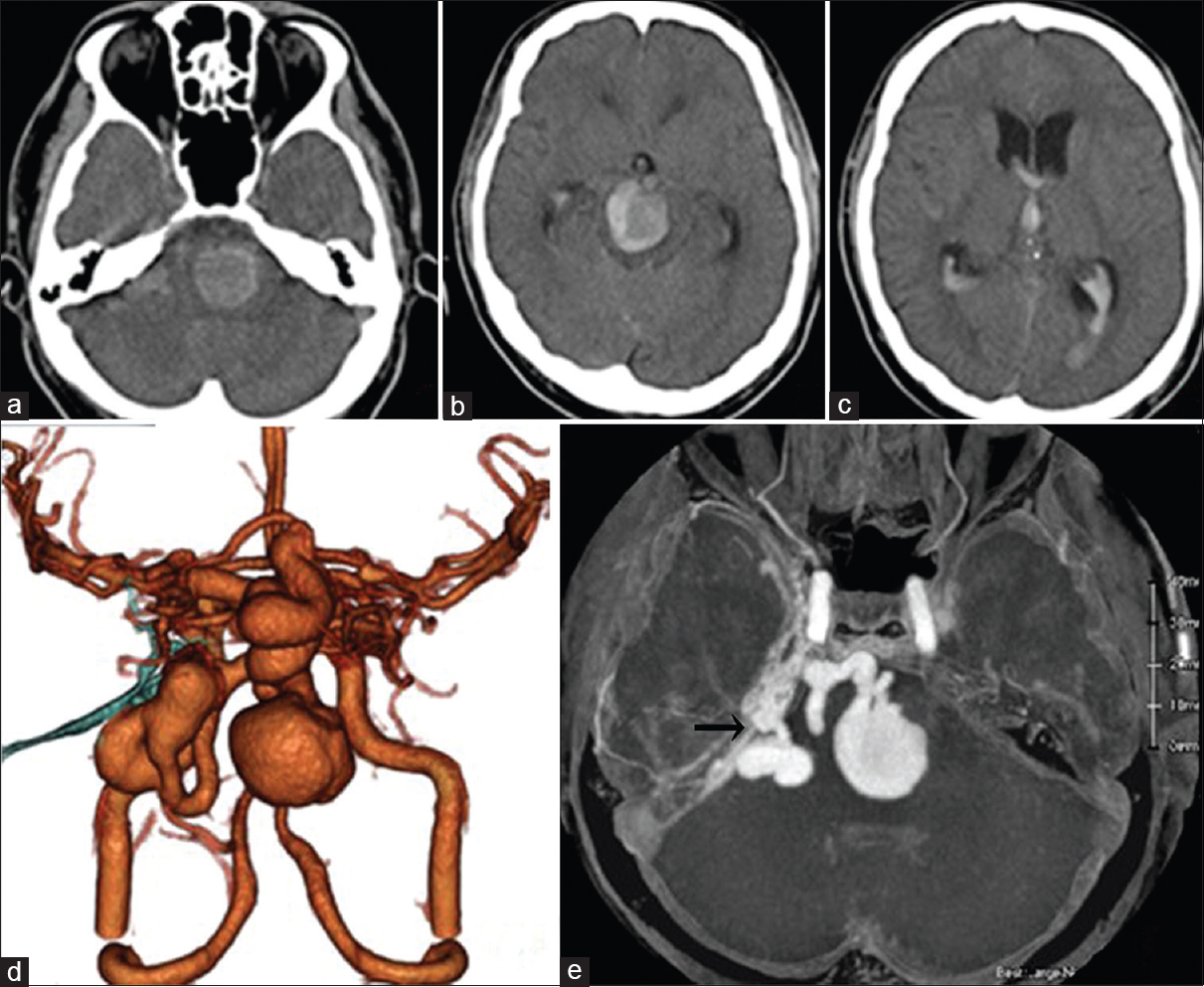
Computed tomography (CT) revealed a very large high-density mass lesion on the pons, midbrain, and cerebellopontine (CP) angle with subarachnoid and intraventricular hemorrhage and acute hydrocephalus [Figure
Figure 2
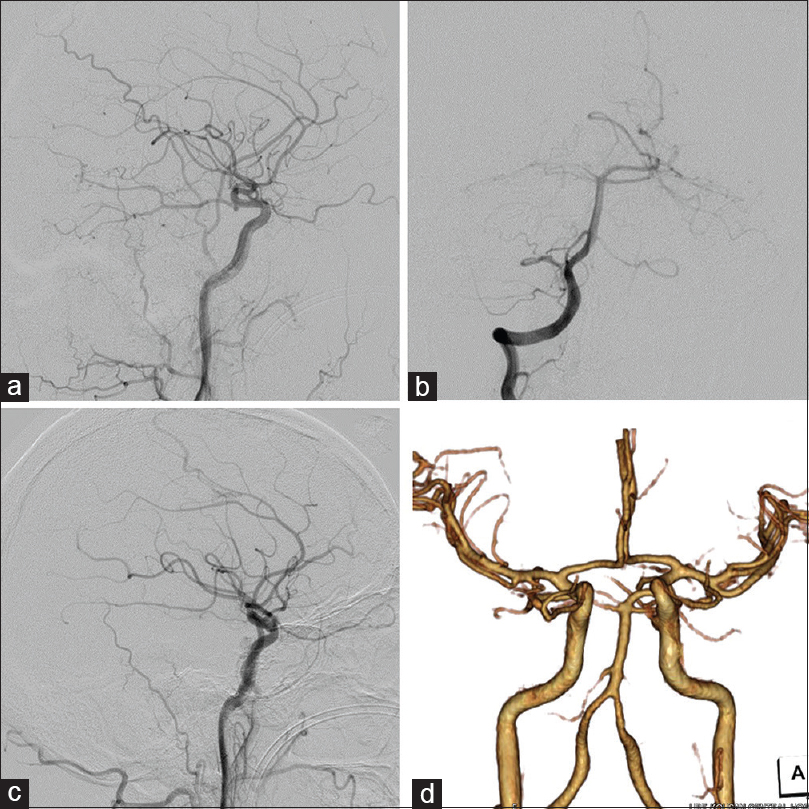
(a) Lateral view on a right external carotid angiogram. The MMA (arrow) and Max. A (arrowhead) are feeding the TDAVF. (b) Anteroposterior view on a right vertebral angiogram. The SCA (arrow) and AICA (arrowhead) are feeding the TDAVF. Lateral view on carotid angiogram (c: right, d: left) showing dilated tentorial arteries (arrows). (e) Simplified scheme relationship among feeing arteries (red), draining veins (blue) and shunting point (asterisk). (f) 3D-CTA after endovascular treatment. The venous pouches of the midbrain became smaller (asterisk). The venous pouch of the CP angle did not change (arrowhead)
The patient underwent immediate lateral ventricular drainage for severe hydrocephalus on the day of admission. Because of a complication related to neurogenic cardiomyopathy, he was in a serious condition. We first attempted endovascular treatment under local anesthesia 2 days after admission. To shrink the venous pouches, transarterial embolization (TAE) of feeders was performed via the right MMA, AMA, and Max. A using 25% n-butyl-2-cyanoacrylate without the penetration of embolus to shunting point. The venous pouches of the midbrain became smaller after embolization, but the size of the pouches in the operative route of the CP angle did not change [
Because the large venous pouch at the CP angle did not allow for an approach at the shunting point [
After surgery, the patient underwent tracheotomy and ventriculoperitoneal shunt for hydrocephalus. His consciousness began to recover and he was withdrawn from the mechanical ventilator. Despite severe neurological deficits including bilateral oculomotor palsy, he had only mild weakness of the upper and lower extremities, and mild cognitive dysfunction. He could walk with limited assistance 6 months after treatment and was grade 3 on the modified Rankin Scale at discharge.
DISCUSSION
TDAVF is a relatively rare but potentially life-threatening condition, with a high risk of hemorrhage. The optimal therapeutic strategy is still debatable. Based on the hemodynamic status of each case, endovascular treatment, surgical disconnection, and stereotactic radiosurgery, or a combination of these procedures, can be used for treatment.[
The retrosigmoid approach was an individualized surgical strategy because the draining vein was the right petrosal vein in our case.[
CONCLUSION
We encountered a severe case of TDAVF with massive hemorrhage, high-flow shunting, and large venous pouches with severe neurological deterioration, which was considered to be difficult to treat without causing serious complications. However, an accurate preoperative diagnosis and appropriate direct surgery with pretreatment of endovascular embolism achieved a satisfactory result. In such a case, proactive treatment is recommended to allow for the best possible outcome, even if the patient is in a moribund condition with hemorrhage.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Awad IA, Little JR, Akarawi WP, Ahl J. Intracranial dural arteriovenous malformations: Factors predisposing to an aggressive neurological course. J Neurosurg. 1990. 72: 839-50
2. Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995. 82: 166-79
3. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995. 194: 671-80
4. Giller CA, Barnett DW, Thacker IC, Hise JH, Berger BD. Multidisciplinary treatment of a large cerebral dural arteriovenous fistula using embolization, surgery, and radiosurgery. Proc (Bayl Univ Med Cent). 2008. 21: 255-7
5. Gross BA, Albuquerque FC, Moon K, McDougall CG. Evolution of treatment and a detailed analysis of occlusion, recurrence, and clinical outcomes in an endovascular library of 260 dural arteriovenous fistulas. J Neurosurg. 2017. 126: 1884-93
6. Guo WY, Pan DH, Wu HM, Chung WY, Shiau CY, Wang LW. Radiosurgery as a treatment alternative for dural arteriovenous fistulas of the cavernous sinus. AJNR Am J Neuroradiol. 1998. 19: 1081-7
7. Halbach VV, Higashida RT, Hieshima GB, Wilson CB, Hardin CW, Kwan E. Treatment of dural fistulas involving the deep cerebral venous system. AJNR Am J Neuroradiol. 1989. 10: 393-9
8. Hiramatsu M, Sugiu K, Hishikawa T, Haruma J, Tokunaga K, Date I. Epidemiology of dural arteriovenous fistula in Japan: Analysis of Japanese registry of neuroendovascular therapy (JR-NET2). Neurol Med Chir (Tokyo). 2014. 54: 63-71
9. Iwamuro Y, Nakahara I, Higashi T, Iwaasa M, Watanabe Y, Hirata E. Tentorial dural arteriovenous fistula presenting symptoms due to mass effect on the dilated draining vein: Case report. Surg Neurol. 2006. p. 511-5
10. Kopitnik TA, Samson DS. Management of dural arteriovenous malformations. Contemp Neurosurg. 1995. 17: 1-8
11. Lawton MT, Sanchez-Mejia RO, Pham D, Tan J, Halbach VV. Tentorial dural arteriovenous fistulae: Operative strategies and microsurgical results for six types. Neurosurgery. 2008. 62: 110-
12. Lewis AI, Tomsick TA, Tew JM, Jr . Management of tentorial dural arteriovenous malformations: Transarterial embolization combined with stereotactic radiation or surgery. J Neurosurg. 1994. 81: 851-9
13. Natarajan SK, Ghodke B, Kim LJ, Hallam DK, Britz GW, Sekhar LN. Multimodality treatment of intracranial dural arteriovenous fistulas in the Onyx era: A single center experience. World Neurosurg. 2010. 73: 365-79
14. Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology. 1969. 93: 1071-8
15. Picard L, Bracard S, Islak C, Roy D, Moreno A, Marchal JC. Dural fistulae of the tentorium cerebelli. Radioanatomical, clinical and therapeutic considerations. J Neuroradiol. 1990. 17: 161-81
16. Soderman M, Pavic L, Edner G, Holmin S, Andersson T. Natural history of dural arteriovenous shunts. Stroke. 2008. 39: 1735-9