- Department of Neurosurgery, Rush University Medical Center, Chicago, United States.
- Department of Neurosurgery, NorthShore University HealthSystem, Evanston, Illinois, United States.
Correspondence Address:
Andrew K. Wong
Department of Neurosurgery, NorthShore University HealthSystem, Evanston, Illinois, United States.
DOI:10.25259/SNI_656_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Andrew K. Wong1, Ricky H. Wong2. Successful treatment of superior sagittal sinus thrombosis after translabyrinthine resection of metastatic neuroendocrine tumor: A case report and review of literature. 25-Nov-2020;11:410
How to cite this URL: Andrew K. Wong1, Ricky H. Wong2. Successful treatment of superior sagittal sinus thrombosis after translabyrinthine resection of metastatic neuroendocrine tumor: A case report and review of literature. 25-Nov-2020;11:410. Available from: https://surgicalneurologyint.com/surgicalint-articles/10415/
Abstract
Background: Postoperative cerebral venous sinus thrombosis (pCVST) after resection of cerebellopontine angle and posterior fossa tumor resections occur almost exclusively in the lateral venous sinuses and are generally asymptomatic. Thrombus extension and involvement of the superior sagittal sinus (SSS) – a serious and potentially devastating complication – are rarely described and, as such, successful treatment for which is still poorly understood. We report a case of pCVST involving the SSS after translabyrinthine approach for resection of a metastatic neuroendocrine tumor (NET), and the first that was successfully treated with anticoagulation therapy.
Case Description: A 40-year-old man presented with headaches, diminished right-sided hearing, and ataxia was found to have a large right-sided cerebellopontine angle (CPA) lesion with extra-axial and possible intraparenchymal invasion. A retrosigmoid craniotomy for debulking and diagnosis was undertaken. Postoperative imaging revealed patent venous sinuses. Pathology confirmed NET. Further imaging revealed a likely pancreatic primary lesion. The patient then underwent subsequent translabyrinthine approach for definitive surgical resection. Postoperative imaging again revealed patent venous sinuses. The patient subsequently developed headaches on postoperative day 10 and was found to have pCVST involving the ipsilateral internal jugular to the SSS. The patient was started on therapeutic heparin with significant improvement in pCVST and symptoms.
Conclusion: Extensive pCVST involving the SSS after CPA and posterior fossa tumor resections is extremely rare. Initial management with anticoagulation can yield promising results and should be initiated early in the clinical course unless otherwise contraindicated.
Keywords: Anticoagulation, Neuroendocrine tumor, Sinus thrombosis, Superior sagittal sinus thrombosis, Translabyrinthine
INTRODUCTION
Cerebral venous sinus thrombosis (CVST) is a known complication after resection of cerebellopontine angle (CPA) and posterior fossa tumors with a reported incidence of 5.2– 38.9%.[
CASE DESCRIPTION
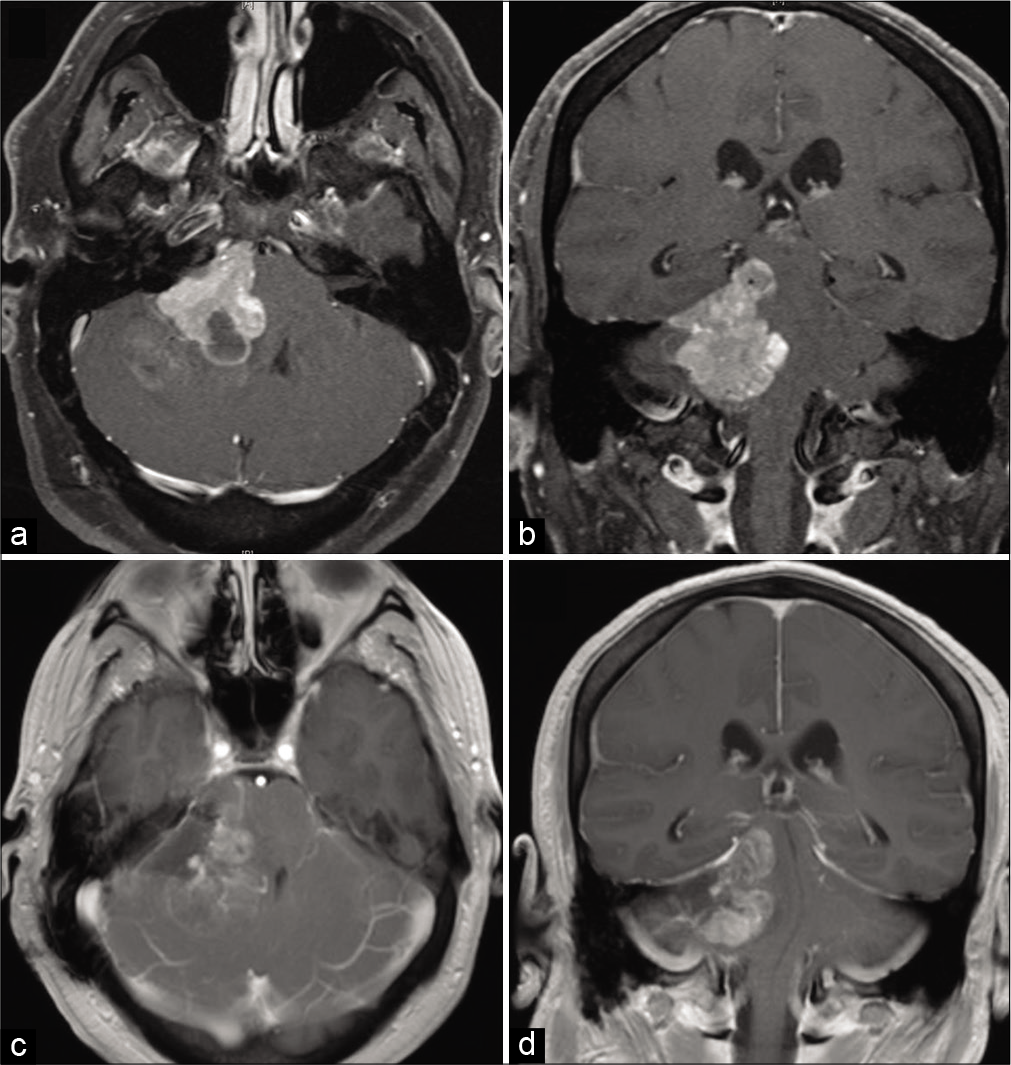
A 40-year-old male with a body mass index (BMI) of 37 kg/m2 but otherwise had no significant medical history presented with symptoms of headaches, diminished hearing on the right, and ataxia. Computed tomography (CT) and subsequent contrasted magnetic resonance imaging (MRI) demonstrated a large right-sided CPA lesion with a circumscribed, extra-axial, contrast-enhancing portion and a diffuse, infiltrating, and sparsely contrast-enhancing portion within the adjacent cerebellum suspicious for intraparenchymal invasion. Patent bilateral codominant lateral venous sinuses were noted on preoperative imaging [
Given the unclear extent of local invasion, the patient underwent a keyhole retrosigmoid craniotomy for diagnostic tumor debulking and subtotal resection. Intraoperative frozen pathology returned as meningioma while final pathological specimen revealed a well-differentiated neuroendocrine tumor (NET). Postoperative contrasted MRI demonstrated patent venous sinuses. CT of the chest, abdomen, and pelvis was inconclusive for a possible primary source. Subsequent Dotatate positron emission tomography scan utilizing gallium-68 revealed increased uptake in the tail of the pancreas.
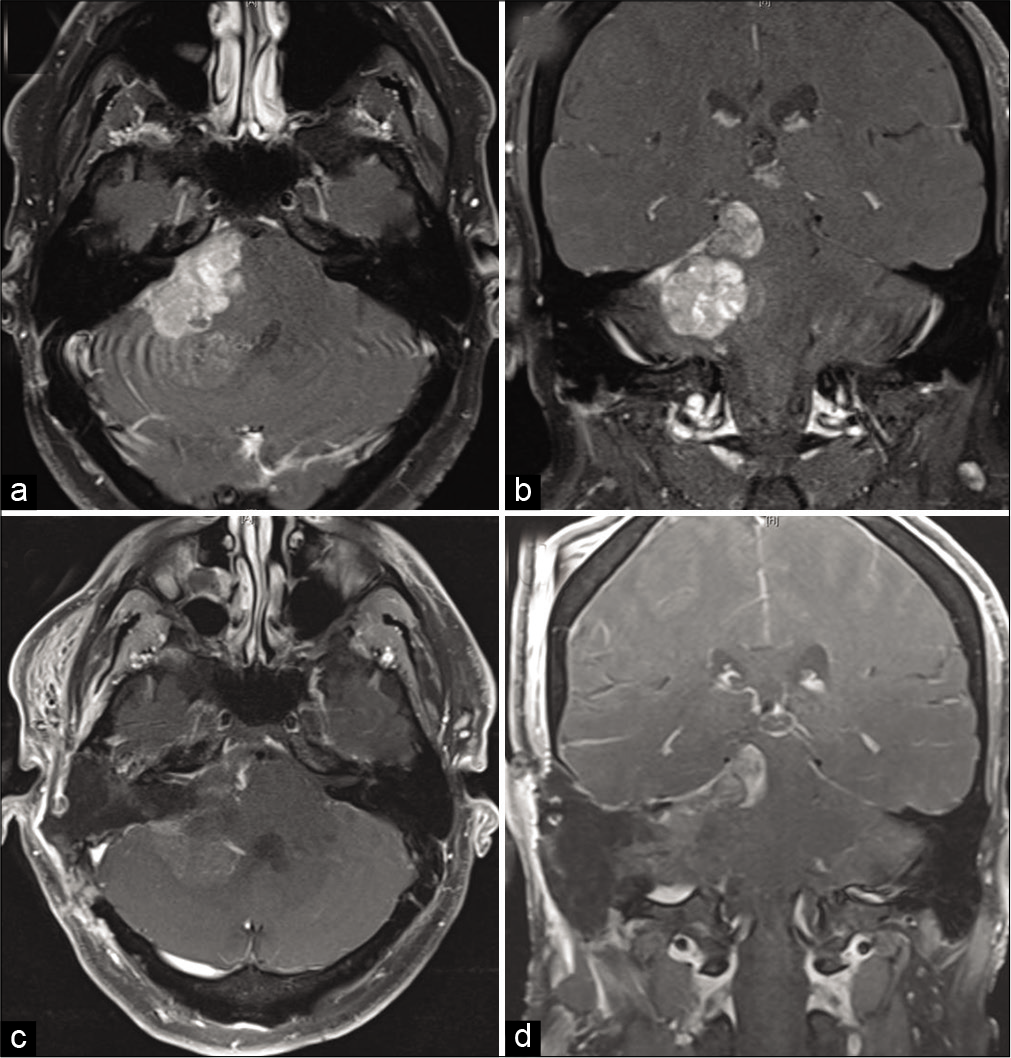
The posterior fossa tumor was initially followed with repeat MRI at 3-month intervals. At 6 months, some growth was noted. After a multidisciplinary discussion, the patient underwent a translabyrinthine approach for definitive surgical resection. While the patient had serviceable hearing, given the tumor extent and risk of hearing loss even with a retrolabyrinthine approach, a translabyrinthine approach was chosen for increased surgical freedom and improved resection. Intraoperative findings were significant for a well-circumscribed extra-axial component that was resected as well as an intraparenchymal portion largely homogenous with surrounding normal brain tissue not amenable for resection. Total operative time was 481 min. No sinus injury was encountered and no fixed retractors were used. Postoperative contrasted MRI again revealed patent venous sinuses [
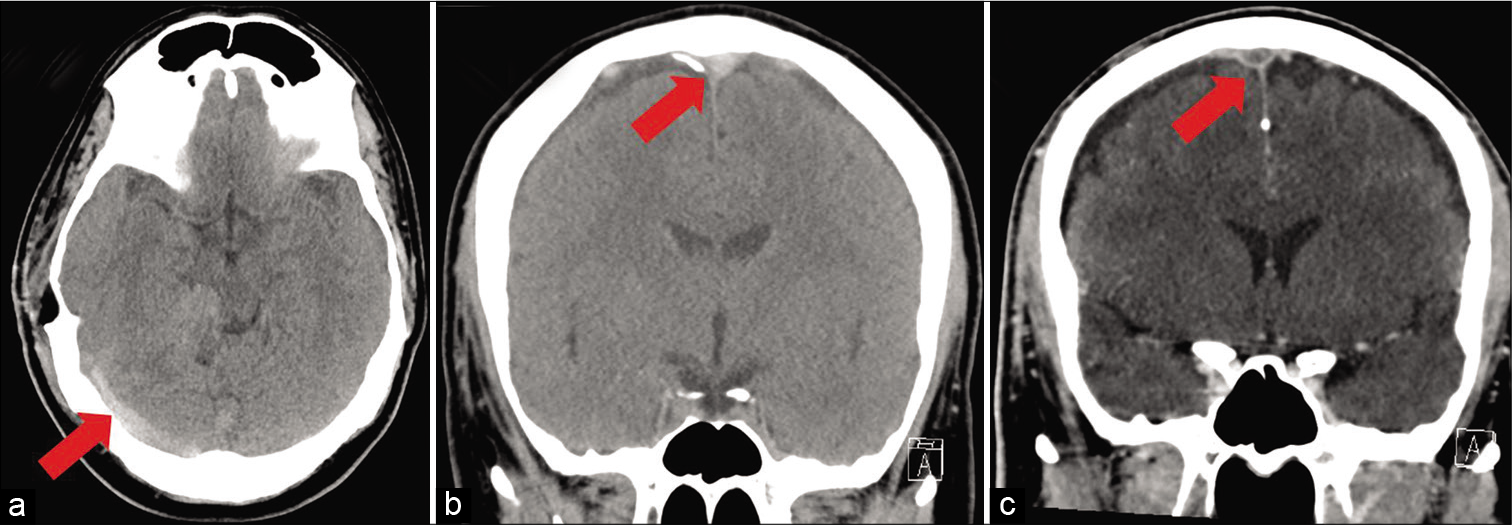
The patient was discharged to an inpatient rehabilitation unit where on postoperative day 10, he developed significant headaches. CT demonstrated hyperdense signal throughout the venous sinuses. CT venogram confirmed the presence of thrombosis within the ipsilateral internal jugular (IJ), sigmoid sinus (SS), transverse sinus (TS), SSS, and superficial cortical veins but without associated hemorrhage [
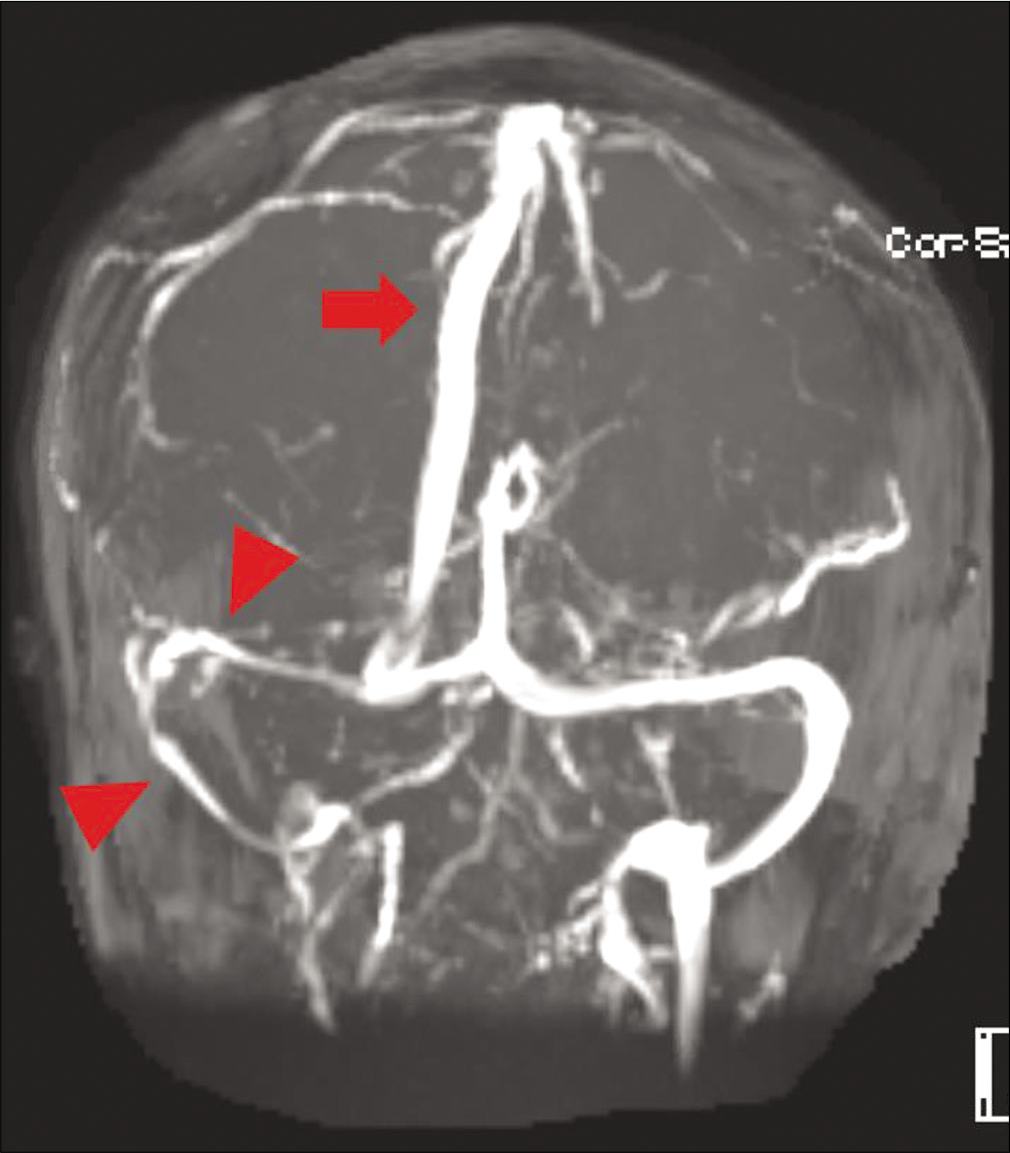
Given the relative proximity to surgery, the patient was started on therapeutic anticoagulation in the form of intravenous heparin with a PTT goal of 50–70 s to preserve reversibility should hemorrhage occur. The patient’s symptoms resolved after treatment was initiated and was ultimately transitioned to apixaban before discharge, which he remained on for 6 months. The patient received adjuvant external beam radiation therapy and started on lanreotide for the treatment of his NET. Magnetic resonance venogram at 6 months demonstrated complete recanalization of the SSS with partial recanalization of the IJ, SS, and TS. The patient remained asymptomatic at 6 months follow-up [
DISCUSSION
NETs are a rare and heterogeneous group of slow-growing tumors derived from enterochromaffin cells that are widely distributed throughout the body. While NETs are known to have malignant potential, they infrequently metastasize to the brain – doing so in only 1.5–5% of cases – and with only two reported cases of neurological symptoms being the first manifestations of metastatic NET disease. Median survival after such diagnosis is 7–10 months.[
CVST in the lateral venous sinuses is a known complication after resection of CPA and posterior fossa tumors. Surgical risk factors for pCVST, particularly in the translabyrinthine approach, include extensive exposure of the sinuses, desiccation from prolong exposure under the operative microscope lamp, direct and thermal injury during bony drilling, mechanical fixed or dynamic retraction, and migration of bone wax used on emissary veins.[
Evidence-based treatment paradigms for pCVST remain lacking, controversial, and largely based on evidence available for the treatment of sCVST. Some advocate diagnostic vigilance and therapeutic anticoagulation or antiplatelet therapy regardless of clinical symptoms as supported by trials involving sCVST.[
As opposed to the lateral sinuses, pCVST involving the SSS after CPA or posterior fossa tumor resection is exceedingly rare and almost always symptomatic with only two reported cases in the literature. Manzoor et al. reported a case of pCVST involving the ipsilateral IJ, SS, TS, and SSS after translabyrinthine resection of an acoustic schwannoma.[
Our patient represents the third reported case of pCVST involving the SSS after resection of CPA or posterior fossa lesion and the first that was successfully treated with anticoagulation therapy. While the patient had an elevated BMI, he otherwise had no identifiable underlying predisposition for hypercoagulability and was not a smoker. It remains unclear if the nature of the skull base metastatic NET played a role in the extensive thrombosis given the unique presentation of both the tumor and thrombosis as well as the paucity of reported cases. Of note, the patient developed symptoms 10 days after surgery while this was outside the immediate postoperative period, making the decision to initiate anticoagulation therapy simpler, it highlights the importance of maintaining a high level of suspicion for CVST in the extended postoperative period as well. Nevertheless, this case provides evidence that anticoagulation therapy can continue to play a central role in the frontline management of extensive pCVST.
CONCLUSION
Although CVST is a known and likely frequent occurrence after resection of CPA and posterior fossa tumors, especially in approaches that require extensive exposure of the venous sinuses, involvement of the SSS is exceedingly rare. Strong evidence of best treatment options in pCVST involving the SSS is lacking and likely unattainable given its extremely low incidence. This report further highlights the importance of maintaining a high level of suspicion for pCVST after such approaches and demonstrates the feasibility in treating extensive thrombosis with therapeutic anticoagulation.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abou-Al-Shaar H, Azab MA, Karsy M, Guan J, Alzhrani G, Gozal YM. Assessment of costs in open surgery and stereotactic radiosurgery for vestibular schwannomas. J Neurosurg. 2018. 131: 561-8
2. Abou-Al-Shaar H, Gozal YM, Alzhrani G, Karsy M, Shelton C, Couldwell WT. Cerebral venous sinus thrombosis after vestibular schwannoma surgery: A call for evidence-based management guidelines. Neurosurg Focus. 2018. 45: E4
3. Apra C, Kotbi O, Turc G, Corns R, Pagès M, SouillardScémama R. Presentation and management of lateral sinus thrombosis following posterior fossa surgery. J Neurosurg. 2017. 126: 8-16
4. Benjamin CG, Sen RD, Golfinos JG, Sen C, Roland JT, McMenomey S. Postoperative cerebral venous sinus thrombosis in the setting of surgery adjacent to the major dural venous sinuses. J Neurosurg. 2019. 2019: 1-7
5. Capecchi M, Abbattista M, Martinelli I. Cerebral venous sinus thrombosis. J Thromb Haemost. 2018. 16: 1918-31
6. Coutinho J, de Bruijn SF, Deveber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Cochrane Database Syst Rev. 2011. 2011: CD002005
7. Guazzo E, Panizza B, Lomas A, Wood M, Amato D, Alalade A. Cerebral venous sinus thrombosis after translabyrinthine vestibular schwannoma-a prospective study and suggested management paradigm. Otol Neurotol. 2020. 41: e273-9
8. Hlatky R, Suki D, Sawaya R. Carcinoid metastasis to the brain. Cancer. 2004. 101: 2605-13
9. Keiper GL, Sherman JD, Tomsick TA, Tew JM. Dural sinus thrombosis and pseudotumor cerebri: Unexpected complications of suboccipital craniotomy and translabyrinthine craniectomy. J Neurosurg. 1999. 91: 192-7
10. Kowoll CM, Kaminski J, Weiß V, Bösel J, Dietrich W, Jüttler E. Severe cerebral venous and sinus thrombosis: Clinical course, imaging correlates, and prognosis. Neurocrit Care. 2016. 25: 392-9
11. Manzoor NF, Ray A, Singer J, Nord R, Sunshine J, Megerian CA. Successful endovascular management of venous sinus thrombosis complicating trans-labyrinthine removal of vestibular schwanomma. Am J Otolaryngol. 2016. 37: 379-82
12. Moore J, Thomas P, Cousins V, Rosenfeld JV. Diagnosis and management of dural sinus thrombosis following resection of cerebellopontine angle tumors. J Neurol Surg B Skull Base. 2014. 75: 402-8
13. Paulo DL, Lopez AM, Jermakowicz WJ, Yu H, Shah H, Konrad PE. Microvascular decompression for trigeminal neuralgia in patients with multiple sclerosis: Predictors of treatment success. World Neurosurg. 2020. 136: e165-70
14. Pavel M, Grossman A, Arnold R, Perren A, Kaltsas G, Steinmüller T. ENETS consensus guidelines for the management of brain, cardiac and ovarian metastases from neuroendocrine tumors. Neuroendocrinology. 2010. 91: 326-32
15. Puppa AD, Tosatto L, Amistà P, Munari M, Scienza R. Transverse sinus thrombosis after posterior fossa surgery for cerebellar tumor treated by endovascular thrombectomy. A case report. Neuroradiol J. 2007. 20: 562-5
16. Sawarkar DP, Verma SK, Singh PK, Doddamani R, Kumar A, Sharma BS. Fatal superior sagittal sinus and torcular thrombosis after vestibular schwannoma surgery: Report of a rare complication and review of the literature. World Neurosurg. 2016. 96: 607.e619-24