- Section of Neurosurgery, Department of Surgery, Aga Khan University, Karachi, Pakistan,
- Department of Surgery, Aga Khan University, Karachi, Pakistan,
- Department of Surgery, Section of Otolaryngology, Aga Khan University, Karachi, Pakistan,
- Digital Health Resource Centre, Aga Khan University Hospital, Karachi, Pakistan,
- Department of Surgery, St. Paul’s Sinus Centre, University of British Columbia, Vancouver, Canada.
Correspondence Address:
Ahsan Ali Khan, Section of Neurosurgery, Department of Surgery, Aga Khan University, Karachi, Pakistan.
DOI:10.25259/SNI_743_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Fahad Zahid1, Ayesha Memon2, Moghira Siddiqui3, Muhammad Hammad Deewani3, Osama Asif4, Amin Javer5, Ahsan Ali Khan1. Successful use of a patient specific 3D-printed biomodel as surgical guide for excision of juvenile nasopharyngeal angiofibroma extending to skull base: A case report. 16-Feb-2024;15:44
How to cite this URL: Fahad Zahid1, Ayesha Memon2, Moghira Siddiqui3, Muhammad Hammad Deewani3, Osama Asif4, Amin Javer5, Ahsan Ali Khan1. Successful use of a patient specific 3D-printed biomodel as surgical guide for excision of juvenile nasopharyngeal angiofibroma extending to skull base: A case report. 16-Feb-2024;15:44. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12757
Abstract
Background: 3-Dimensional (3D) printing has proven its role in various fields. Recently, 3D printing has also been introduced in the otolaryngology domain. The nasopharynx, paranasal sinuses, and the anterior skull base have a complex anatomy. Critical structures must be delicately protected and preserved during a surgical procedure. It is, therefore, very important for the surgeon to have an excellent spatial understanding of the complex surgical field that is being traversed.
Case Description: Our case is of a 19-year-old male with a 2-month history of recurrent epistaxis, nasal blockage, and headache. Based on the computed tomography scan and the clinical presentation, the patient was diagnosed with juvenile nasopharyngeal angiofibroma. The patient underwent angioembolization of the tumor followed by endoscopic surgical resection. The patient remained stable postoperatively and demonstrated a good recovery in the follow-up visit with no signs of cranial deficits. This case report highlights the use of a patient-specific 3D-printed biomodel to visualize this rare tumor of the nasopharynx. The benefits of using the model in surgical planning, patient education, and resident training are reported. We found that the ability to visualize the tumor on a tangible model, viewing its actual size in relation to the adjacent anatomy and all the structures associated with it, greatly enhances the surgeon’s capacity to tackle such a difficult tumor endoscopically.
Conclusion: Incorporating 3D-printed biomodels in surgical practice should result in improved outcomes for the patients.
Keywords: 3D printing, Angiofibroma, Biomodel, Simulation, Skull base
INTRODUCTION
Juvenile nasopharyngeal angiofibroma is a rare tumor accounting for approximately 0.05% of head and neck tumors.[
In neurosurgery, presurgical planning using 3D-printed brain tumor models ensures safe and successful surgeries, especially for less experienced surgeons.[
CASE PRESENTATION
A 19-year-old male patient presented to our hospital with a chief complaint of recurrent nasal bleeding over two months. He also complained of progressive nasal blockage, continuous nasal discharge, and occasional headaches throughout the past few weeks, with a history of similar complaints two years previously. He had no other comorbidities, nor was he taking any regular medications. His surgical history was significant for radiotherapy of the tumor in 2019 and an attempted resection of a nasal mass in 2020. His family history and physical examination were unremarkable. His initial relevant laboratory workup was reported to be normal.
CT findings
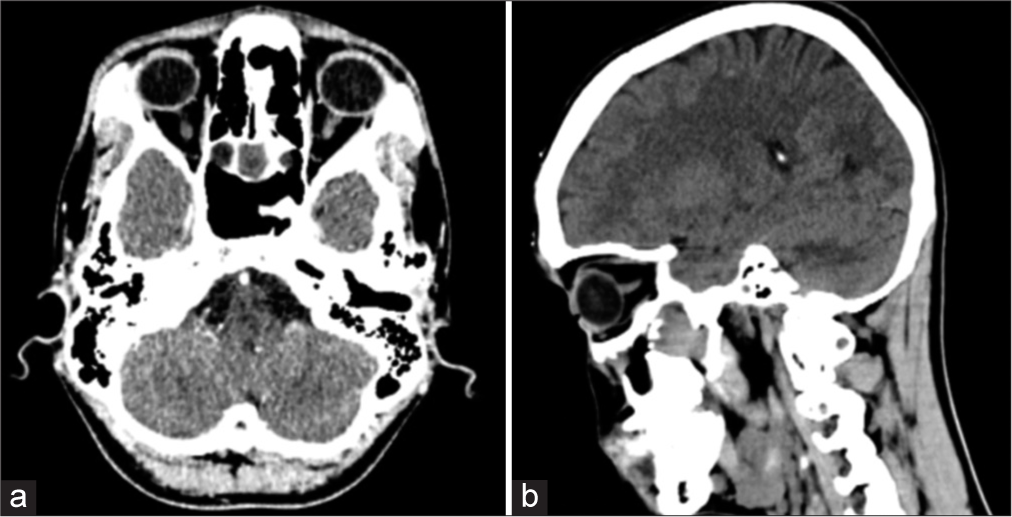
His CT scan showed a lobulated, enhancing soft-tissue mass in the region of the right pterygopalatine fissure and bilateral posterior ethmoid sinuses, anterior to the medial pterygoid plate with extension into both sphenoid sinuses posteriorly and the right maxillary sinus laterally. Arbitrarily, the mass measured approximately 29 mm × 29 mm in the craniocaudal and transverse dimensions. The enhancing soft-tissue mass anterior to the right pterygoid plates measured approximately 18 mm × 12 mm. The right maxillary sinus was not visualized, status-post intervention. The brain showed normal gray and white matter differentiation. No acute intracranial hemorrhage, established infarct, or mass effect was seen. There was no hydrocephalus or shift of the midline structures. Ventricles and basal cisterns appeared normal. His non-contrast CT scan images are shown in
The case was thoroughly reviewed, and he was managed along the lines of a recurrent angiofibroma. If not treated, the patient would have suffered from persistent nose bleeds, and the tumor would invade into the cranium. Due to the complex site of the tumor, neurosurgery was also consulted. The family was extensively counseled, and after informed consent, he was planned for an endonasal endoscopic resection of the juvenile angiofibroma post angioembolization.
3D biomodel development
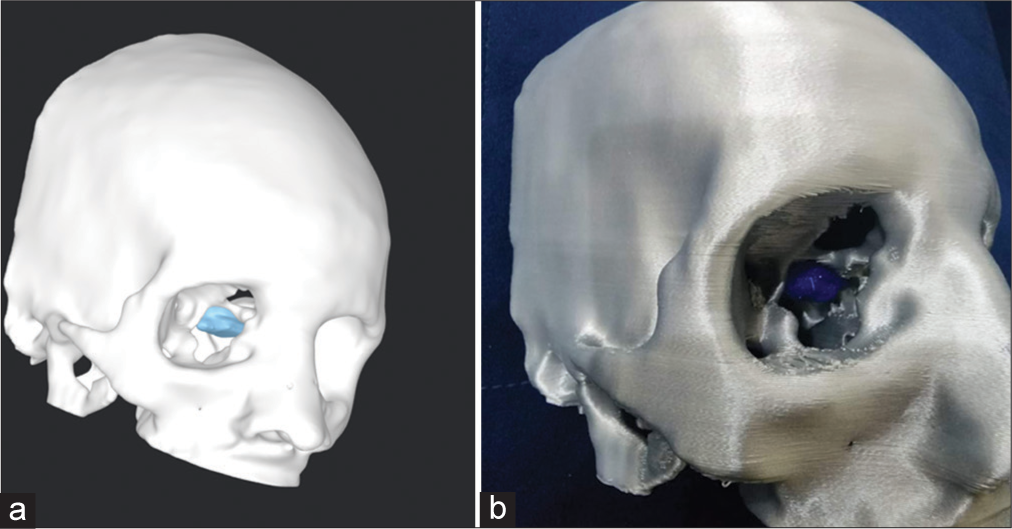
A team of Biomedical Engineers was involved in this case for the development of a patient-specific 3D-printed biomodel [
Angioembolization
A 4 French vascular access sheath was placed in the right femoral artery, and through this access, bilateral external carotid arteries were cannulated, and angiograms were performed. The angiogram revealed a vascular blush in the posterior nasal region corresponding to the lesion seen on diagnostic imaging. This was predominantly supplied by bilateral internal maxillary artery branches (right>Left). The supplying branches were selectively cannulated and embolized using polyvinyl alcohol particles through a microcatheter. Post embolization run showed a significant reduction in the arterial supply of the lesion.
Tumor resection
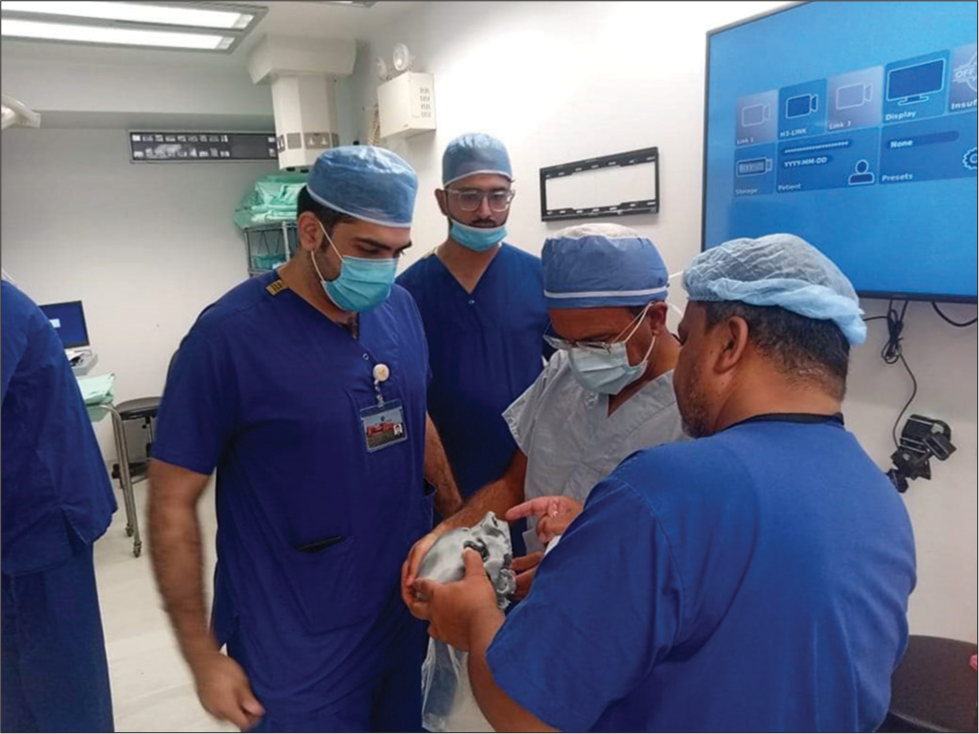
Two days after angioembolization, the patient underwent endoscopic endonasal excision of the angiofibroma under general anesthesia with a cover of hypotensive drugs to minimize bleeding. The 3D biomodel was used by the surgical team, preoperatively [
Postoperative care
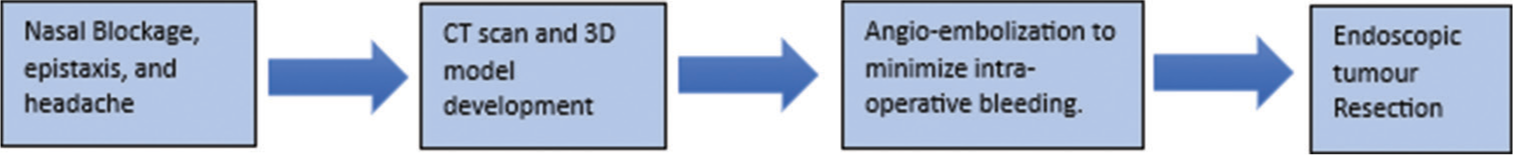
The patient was closely monitored for the next two days postoperatively. He remained vitally and clinically stable. Postoperatively, his extraocular movements, pupillary reflex, and higher mental functions were thoroughly examined and found to be normal. On a follow-up visit to the clinic, he showed promising recovery with no signs of any cranial deficits. The summary of the patient’s management is described in
DISCUSSION
In the 5th century BC, Hippocrates first described the tumor which was later termed “angiofibroma” in 1940 by Friedberg. The tumor, over time, has gained the name “Juvenile Nasopharyngeal Angiofibroma” due to its predisposition in the adolescent age group.[
A CT scan, MRI, and angiography are the main radiological tools used to determine the position, size, extent, erosion into the surrounding bony structures, and invasion through the various foramina and fissures. Bony involvement is best demonstrated on CT scan, intracranial spread on MRI, and feeding vessels can be best identified on angiography, which can also be used for preoperative angioembolization to reduce hemorrhagic complications. The arterial supply of the tumor is largely from the distal internal maxillary artery branches, namely, the sphenopalatine, descending palatine, and posterior superior alveolar branches.[
The ability to obtain a 3D-printed model where a detailed knowledge of patient-specific anatomy can be physically observed aids in planning the best route of surgery. It not only gives the surgeon a better modality to plan for the best surgical approach but can also enhance safety by minimizing the risk of a complication during the actual surgical procedure. In our case, the surgeon was able to successfully use the tactile dimensions of the model and plan the endoscopic approach for the angiofibroma. Due to the recurrent nature and complexity of the tumor in our case, this added modality in the planning phase of the surgery significantly improved our capacity to resect the tumor completely without any residual tissue, which could have resulted in a recurrence.
In a collaborative attempt between Otolaryngology and Neurosurgery to remove this complex angiofibroma, which was adhered to the dura mater, a new modality was utilized. Nowadays, 3D-printed models are utilized not only for orthopedics surgeries but also play a major role in skull base tumor resections, reconstructive maxillofacial surgeries, and cardiac surgeries as well. Multiple different types of 3D-printed models allow us to differentiate the normal tissue from the tumor, thus allowing the surgeon to review the tumor margins more thoroughly with its anatomical surrounding structures. 3D-printed models specific to the patient mimic the complex and distorted anatomy of the patient. Numerous workshops with 3D-printed skulls have been carried out and it is found to be a useful tool in individualized surgical planning for endoscopic surgeries.[
Preoperative planning
A 3D model may help increase the accuracy and reduce the duration of surgery, which would, in turn, reduce intraoperative risks and decrease anesthesia time and exposure to ionizing radiation. Studies have demonstrated benefits in patient outcomes with the incorporation of 3D models.[
According to a case report on using 3D-printed models in endoscopic sinus surgery, the patient-specific 3D model aided in improved visualization of tumor size and its relation to the surrounding anatomy.[
Patient education
Good patient satisfaction requires that patients have all their concerns addressed. This often means that they want to understand their pathology. 3D-printed models serve as an excellent tool to serve that purpose. In the case of tumors, these models help patients understand where the tumor lies and why a particular intervention is being carried out in a particular manner. Sander et al.[
Surgical teaching
With advances in technology, there is a natural and parallel increase in the complexity, accuracy, and approaches to surgical planning. Effective surgical training is enhanced when utilizing simulation on 3D models for junior trainees to learn the essential skills. Surgery of the paranasal sinuses and the anterior skull base requires expertise in protecting critical structures such as the optic nerve, brainstem, brain, and carotid arteries. A workshop conducted by Narayanan et al., found that simulating endoscopic sinus and skull base surgeries helped trainees learn the crucial steps of the procedure, such as image-guided navigation and the use of power tools for endoscopic drilling.[
CONCLUSION
Juvenile nasopharyngeal angiofibroma is a rare, benign tumor that is mainly prevalent in the adolescent male population. The main presentation of the affected individual varies from unilateral to bilateral nasal blockage to severe epistaxis. A contrast CT scan is the first and most accurate radiological method to check for the tumor’s size and extension, helping in staging the tumor to denote the best possible surgical approach. Angiography, along with angioembolization, is the best collateral procedure done before surgery to minimize blood loss and consequently reduce fatal risk factors. In our case, we created and utilized a 3D-printed biomodel to further enhance 3D visualization of the tumor, allowing us to practice and identify the best approach for surgery, thereby minimizing operating time complications and ensuring faster recovery and reduced hospital stay for our patient. It is important to note that although not 100% accurate, the 3D-printed biomodel enables the surgeon to plan the approach for tumor excision with high accuracy, thereby minimizing unfavorable consequences for the excision of a tumor with a high risk of bleeding.
Authors’ contributions
AM wrote the initial draft of the manuscript. FZ co-wrote and edited the manuscript. MS, MHD, and AAK conceptualized and designed the framework of the study. OA provided technical expertise with regard to generating a 3D patient-specific biomodel on the computer and then printing it using a 3D printer. AJ led the surgical team. MHD was involved in managing the patient. MS, AJ, and AAK participated in the surgery and provided key edits to the manuscript based on their expertise. All authors reviewed and approved the final manuscript.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the present study.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We express our gratitude to the Ideas and Innovation Division at the Department of Surgery and the Technology Innovation Support Centre for providing the necessary resources to carry out this study.
References
1. Atalar MH, Solak O, Müderris S. Juvenile nasopharyngeal angiofibroma: Radiologic evaluation and pre-operative embolization. KBB Forum. 2006. 5: 56-61
2. Budu V, Bulescu I, Mogoantă CA. Particular aspects in endoscopic surgery for juvenile nasopharyngeal angiofibromas. Case reports and review of literature. Rom J Morphol Embryol. 2013. 54: 867-70
3. Essayed WI, Unadkat P, Hosny A, Frisken S, Rassi MS, Mukundan S. 3D printing and intraoperative neuronavigation tailoring for skull base reconstruction after extended endoscopic endonasal surgery: Proof of concept. J Neurosurg. 2018. 130: 248-55
4. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE guidelines: Consensus-based clinical case reporting guideline development. Glob Adv Health Med. 2013. 2: 38-43
5. Huang X, Liu Z, Wang X, Li XD, Cheng K, Zhou Y. A small 3D-printing model of macroadenomas for endoscopic endonasal surgery. Pituitary. 2019. 22: 46-53
6. Jimenez JE, Shaffer AD, Hammersley E, Ghodadra A, Stapleton AL. Use of patient-specific 3D printed models in pre-operative counseling for pediatric skull base surgery. Int J Pediatr Otorhinolaryngol. 2023. 171: 111655
7. Low CM, Morris JM, Price DL, Matsumoto JS, Stokken JK, O’Brien EK. Three-dimensional printing: Current use in rhinology and endoscopic skull base surgery. Am J Rhinol Allergy. 2019. 33: 770-81
8. Mallon A, Farnan T. A case report detailing the use of 3D printing technology in surgical planning and decision making in ENT surgery-an axial 3D first in Northern Ireland. Int J Surg Case Rep. 2021. 87: 106407
9. Narayanan V, Narayanan P, Rajagopalan R, Karuppiah R, Rahman ZA, Wormald PJ. Endoscopic skull base training using 3D printed models with pre-existing pathology. Eur Arch Otorhinolaryngol. 2015. 272: 753-7
10. Oishi M, Fukuda M, Yajima N, Yoshida K, Takahashi M, Hiraishi T. Interactive presurgical simulation applying advanced 3D imaging and modeling techniques for skull base and deep tumors. J Neurosurg. 2013. 119: 94-105
11. Panesar SS, Magnetta M, Mukherjee D, Abhinav K, Branstetter BF, Gardner PA. Patient-specific 3-dimensionally printed models for neurosurgical planning and education. Neurosurg Focus. 2019. 47: E12
12. Rogers DJ, Bevans SE, Harsha WJ. Endoscopic resection of juvenile nasopharyngeal angiofibroma. Adv Otorhinolaryngol. 2012. 73: 132-6
13. Sander IM, Liepert TT, Doney EL, Leevy WM, Liepert DR. Patient education for endoscopic sinus surgery: Preliminary experience using 3D-printed clinical imaging data. J Funct Biomater. 2017. 8: 13
14. Vlăescu AN, Ioniţă E, Ciolofan MS, Mogoantă CA, Voiosu C, Rusescu A. Current approach of juvenile nasopharyngeal angiofibroma: A case series. Rom J Morphol Embryol. 2022. 63: 105-11
15. Wake N, Rude T, Kang SK, Stifelman MD, Borin JF, Sodickson DK. 3D printed renal cancer models derived from MRI data: Application in pre-surgical planning. Abdom Radiol (NY). 2017. 42: 1501-9