- Department of Surgery, Navamindradhiraj University, Bangkok, Thailand.
Correspondence Address:
Kitiporn Sriamornrattanakul, Department of Surgery, Navamindradhiraj University, Bangkok, Thailand.
DOI:10.25259/SNI_999_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kitiporn Sriamornrattanakul, Nasaeng Akharathammachote, Somkiat Wongsuriyanan. Suprafascial dissection for pterional craniotomy to preserve the frontotemporal branch of the facial nerve with less temporal hollowing. 16-Nov-2021;12:559
How to cite this URL: Kitiporn Sriamornrattanakul, Nasaeng Akharathammachote, Somkiat Wongsuriyanan. Suprafascial dissection for pterional craniotomy to preserve the frontotemporal branch of the facial nerve with less temporal hollowing. 16-Nov-2021;12:559. Available from: https://surgicalneurologyint.com/surgicalint-articles/11230/
Abstract
Background: To protect the frontotemporal branch of the facial nerve (FTFN) when performing pterional craniotomy, several reports suggest the subfascial or interfascial dissection technique. However, the reports of postoperative frontalis paralysis and temporal hollowing, which are common complications, were relatively limited. This study reports the incidence of postoperative frontalis paralysis and temporal hollowing after pterional craniotomy using the suprafascial and interfascial techniques.
Methods: Patients who underwent pterional craniotomy, using the suprafascial technique (leaving the muscle cuff and not leaving the muscle cuff) and the interfascial technique, between November 2015 and September 2018 were retrospectively evaluated for postoperative frontalis paralysis and temporal hollowing using Chi-squared/ Fisher exact test.
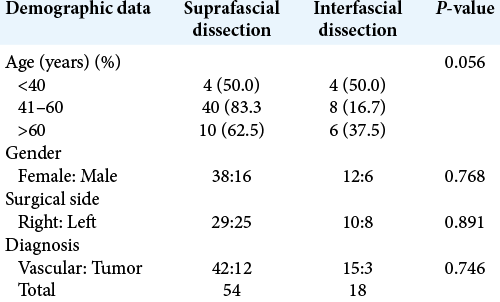
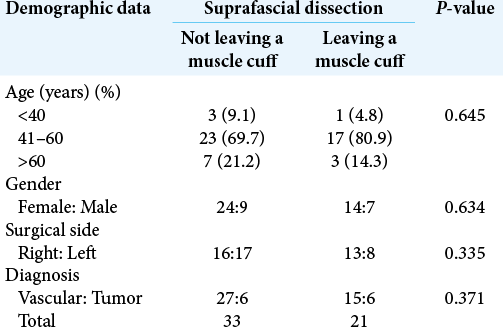
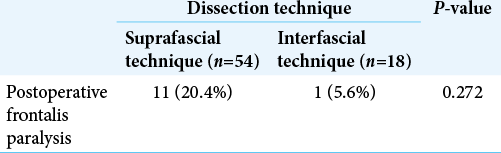
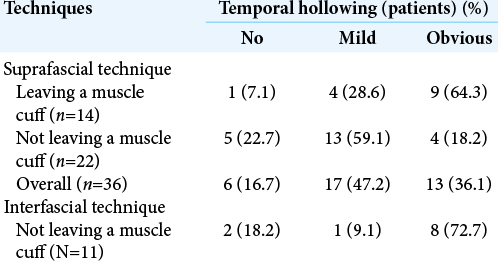
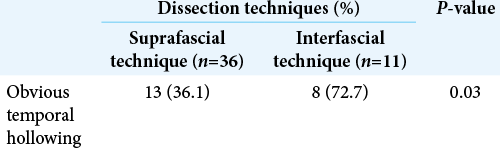
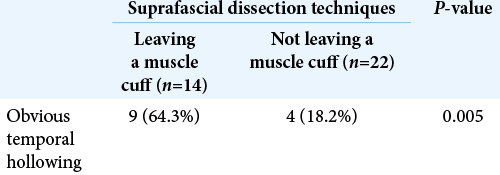
Results: Seventy-two patients underwent pterional craniotomy, using the suprafascial technique in 54 patients (leaving the muscle cuff in 21 patients and not leaving the muscle cuff in 33 patients) and the interfascial technique in 18 patients. Eleven patients (20.4%) in the suprafascial group and 1 patient (5.6%) in the interfascial group developed transient frontalis paralysis (P = 0.272). No permanent frontalis paralysis was observed. Obvious temporal hollowing occurred in 18.2% of patients in the suprafascial group without the muscle cuff, in 64.3% of patients in the suprafascial group with the muscle cuff, and in 72.7% of patients in the interfascial group (P = 0.003).
Conclusion: The suprafascial dissection technique does not cause permanent injury of the FTFN, and this approach results in a significantly lower incidence of postoperative temporal hollowing than interfascial dissection, especially without leaving a temporalis muscle cuff.
Keywords: Facial nerve preservation, Frontalis paralysis, Pterional craniotomy, Suprafascial dissection, Temporal hollowing
INTRODUCTION
The two-layer technique of scalp flap creation for pterional craniotomy gives better exposure of the pterion and sphenoid rim compared with the one-layer technique (myocutaneous flap) wherein the muscle bulge blocks the visualization of these area.[
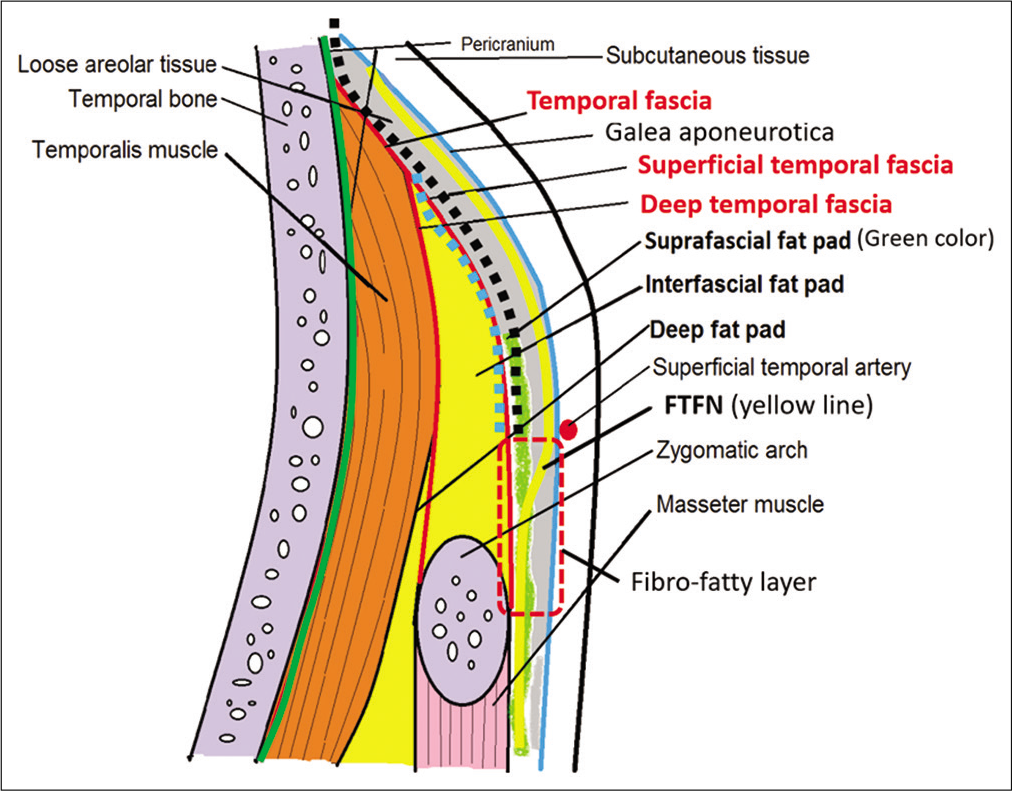
Figure 1:
Schematic depiction of the anatomy of the temporal area in the coronal section and course of frontotemporal branch of the facial nerve. The area of the fibro-fatty layer is shown in the red dashed box. The planes of suprafascial dissection and interfascial dissection are indicated by the black dotted line and blue dotted line, respectively.
Therefore, many researchers advise against dissection in the plane between the galea aponeurotica and the temporal fascia, known as suprafascial (subgaleal) dissection. Subsequently, several techniques were developed to protect the FTFN during pterional craniotomy such as interfascial and subfascial dissection techniques [
In 1998, Salas et al. studied the anatomy of the FTFN in cadavers and suggested that when only the zygomatic process of the frontal bone needs to be exposed (pterional craniotomy), it is not necessary to perform interfascial dissection and that interfascial dissection is indicated when the zygomatic bone or zygomatic process of the temporal bone needs to be exposed (zygomatic arch osteotomy or orbitozygomatic osteotomy). However, the clinical results were not reported.[
Postoperative frontalis paralysis and temporal hollowing are also common complications after pterional craniotomy. Temporal hollowing is reported to occur after 87–100% of temporal craniotomies.[
This study aims to show the incidence of postoperative frontalis paralysis and temporal hollowing after pterional craniotomy using the suprafascial and interfascial dissection techniques.
MATERIALS AND METHODS
Between November 2015 and September 2018, we retrospectively reviewed patients who underwent pterional craniotomy using suprafascial and interfascial dissection techniques for cerebrovascular or tumor surgery.
Exclusion criterion was (1) Bilateral pterional craniotomy; (2) Previous ipsilateral facial weakness; (3) Orbitozygomatic osteotomy; (4) Ipsilateral re-craniotomy; (5) Extensive removal of lateral orbital wall; (6) Indirect bypass using temporalis muscle; (7) Muscle patch graft or fascia taken from the ipsilateral temporalis muscle; and (8) Patients loss to follow-up from the outpatient clinic within 9 months after surgery. Most procedures were performed with suprafascial dissection. The indication for interfascial dissection was to teach residents this widely used technique.
Surgical techniques for suprafascial dissection
Scalp incision with superficial temporal artery preservation was performed just above the root of the zygoma [
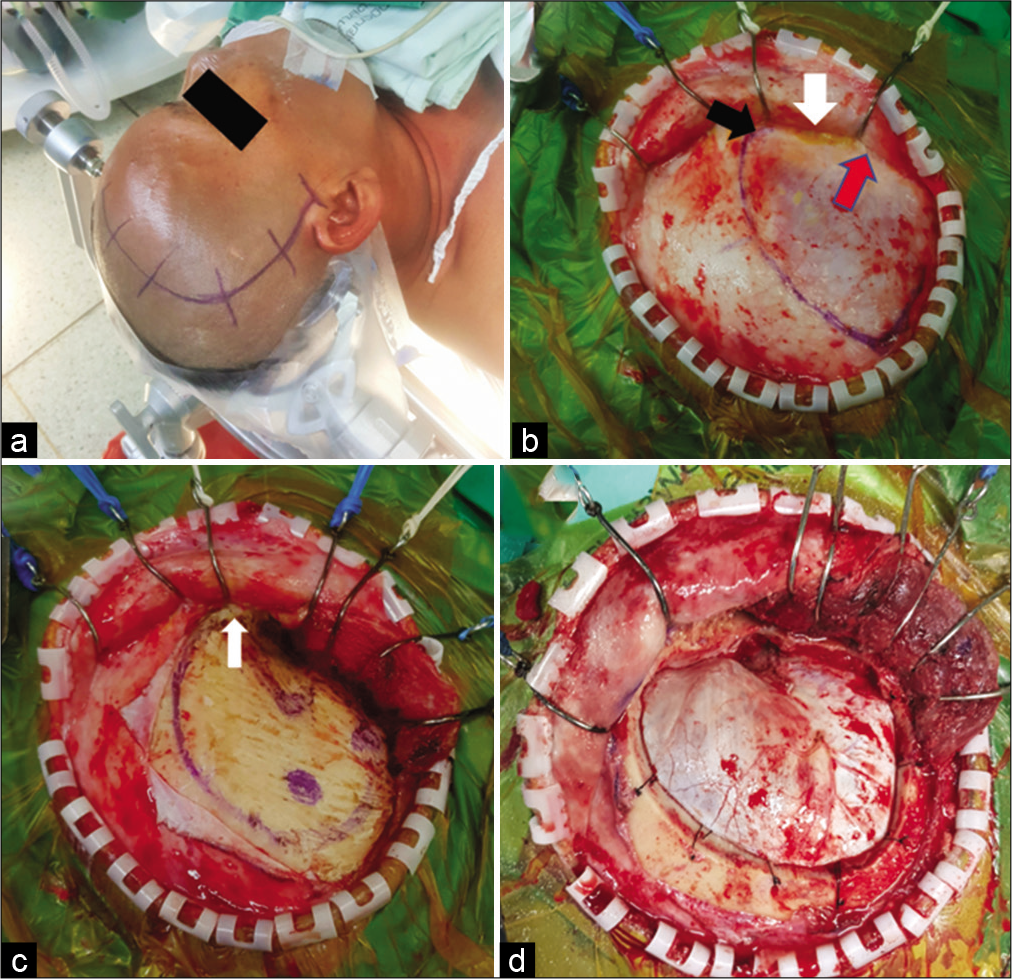
Figure 2:
The suprafascial dissection without muscle cuff for right pterional craniotomy. (a) Skin incision. (b) Scalp flap was elevated through the suprafascial plane. Suprafascial fat pad (white arrow) was elevated together with scalp flap until the frontozygomatic process (black arrow) was reached without violation to the fibro-fatty adhesion (red arrow). The temporal line was marked with marker pen. (c) The temporalis muscle was elevated from the skull without a muscle cuff. The temporal fascia was cut along the frontozygomatic process (white arrow). (d) Frontotemporal craniotomy was performed.
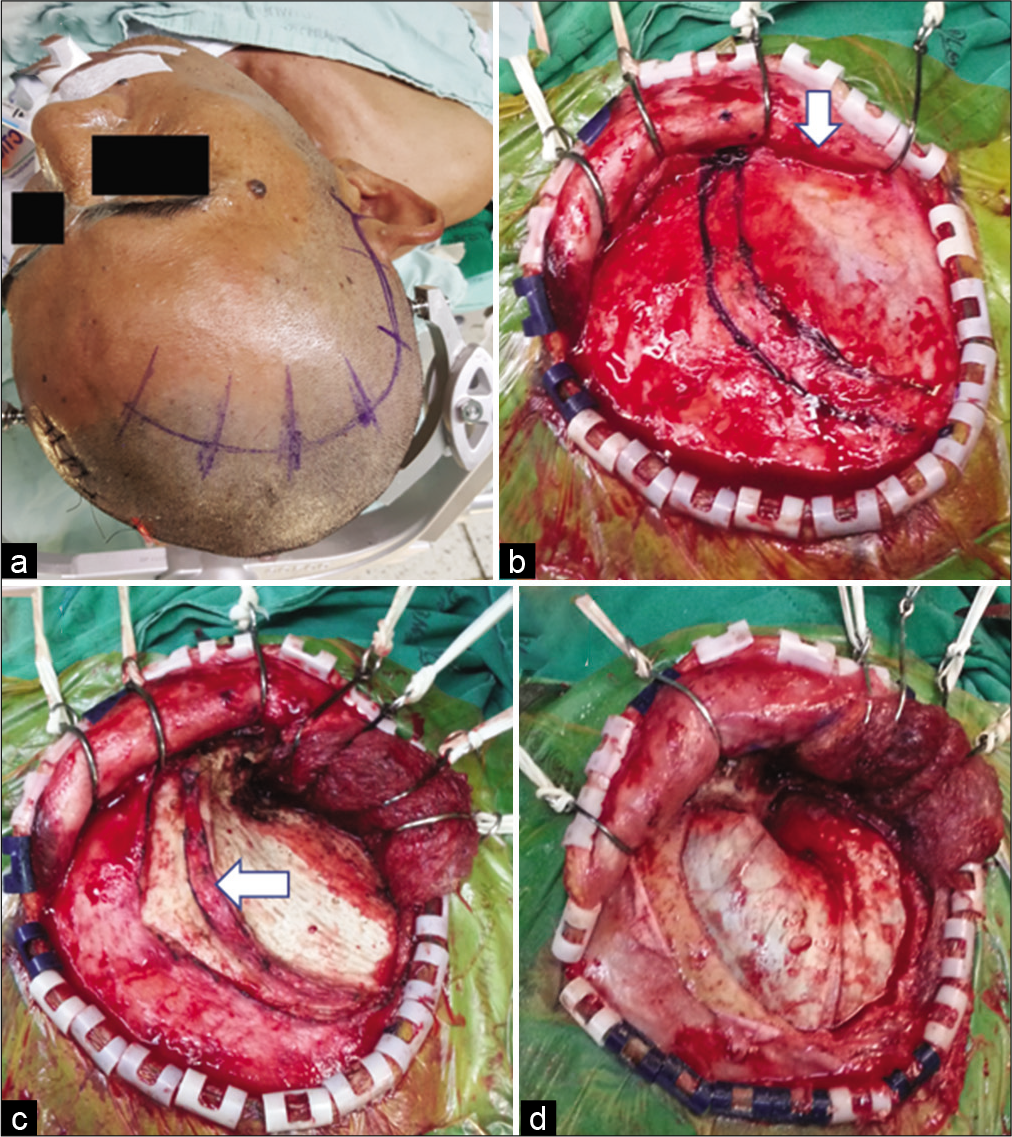
Figure 3:
The suprafascial dissection with the muscle cuff for right pterional craniotomy. (a) Skin incision. (b) Scalp flap was elevated through the suprafascial plane. Suprafascial fat pad (arrow) was elevated together with the scalp flap. The temporal line and temporal muscle cuff were marked with marker pen. (c) The temporalis muscle was elevated from the skull, leaving the muscle cuff (arrow) to the skull. (d) Frontotemporal craniotomy was performed.
Surgical techniques for interfascial dissection
The technical steps were same as for the suprafascial dissection, without leaving the muscle cuff [
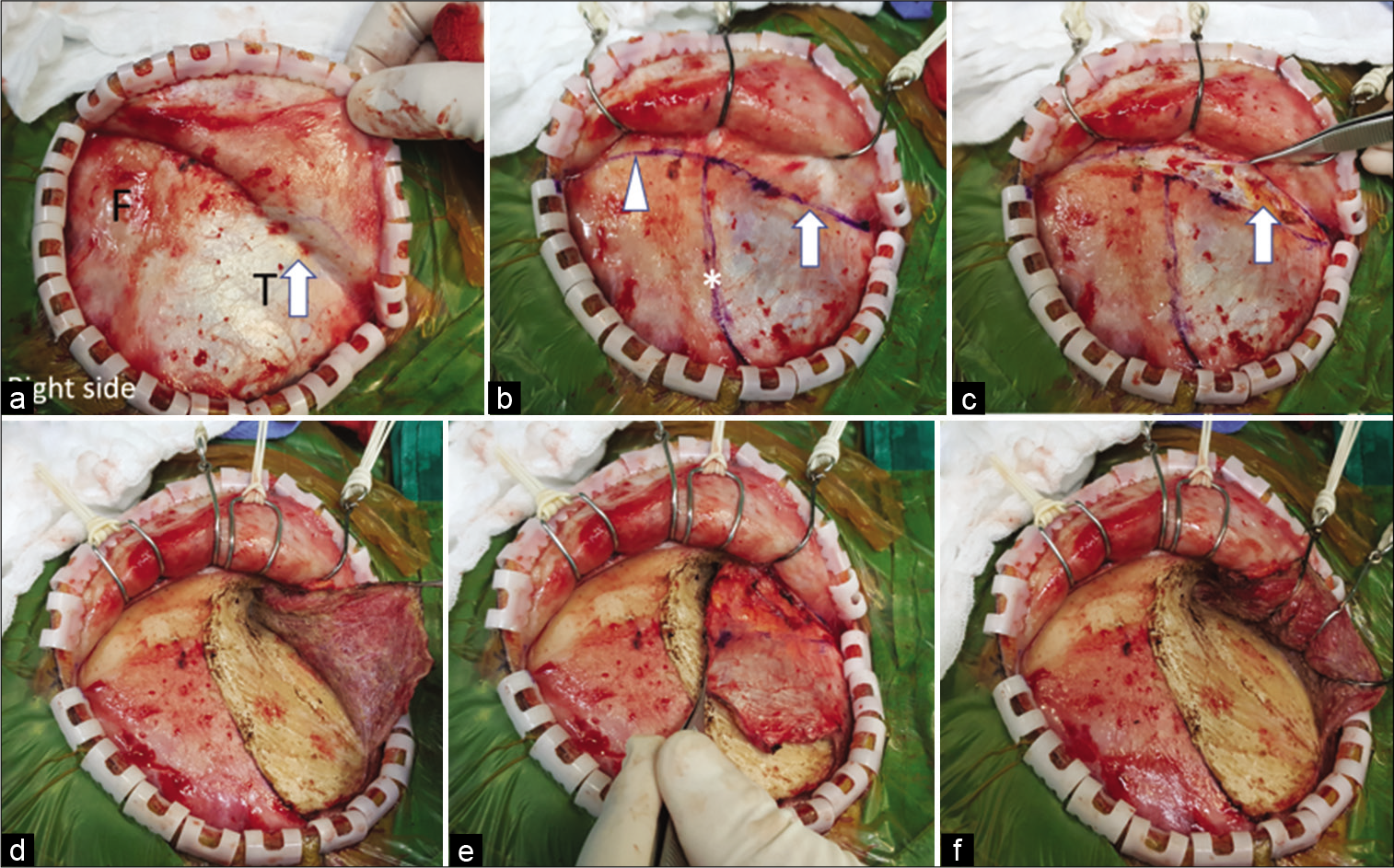
Figure 4:
The interfascial dissection for right pterional craniotomy. (a) The scalp flap was elevated through the suprafascial plane until the interfascial fat pad (arrow) was seen through the superficial temporal fascia. (b) The planned fascia (arrow) and pericranial incision (arrow head) were marked with marker pen. The temporal line (asterisk) was also marked. (c) The superficial temporal fascia (grasped with forceps) and pericranium were incised to expose the interfascial fat pad (arrow). (d) The temporalis muscle was incised along the temporal line. (e) The temporalis muscle was elevated from the skull without the muscle cuff. (f) The temporalis muscle was retracted posteroinferiorly with scalp hooks. F: Frontal, T: Temporal.
Outcome assessment
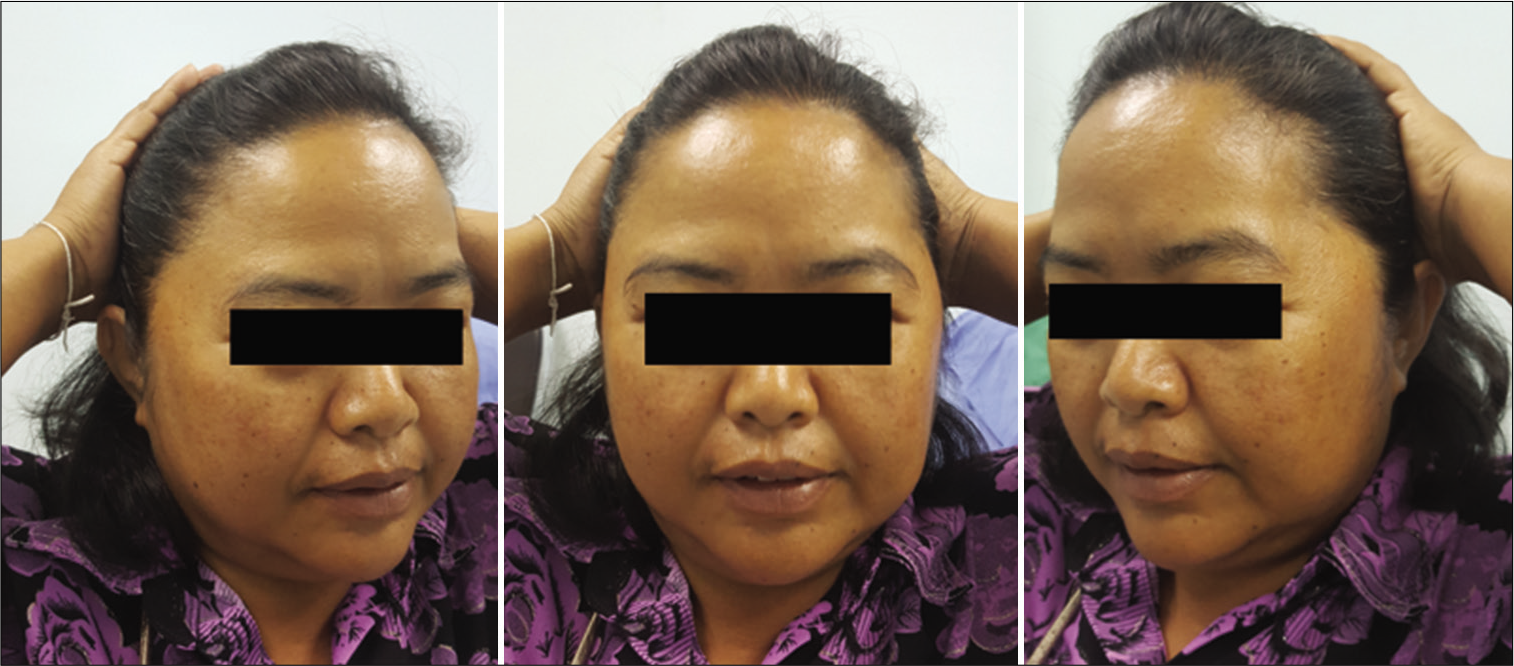
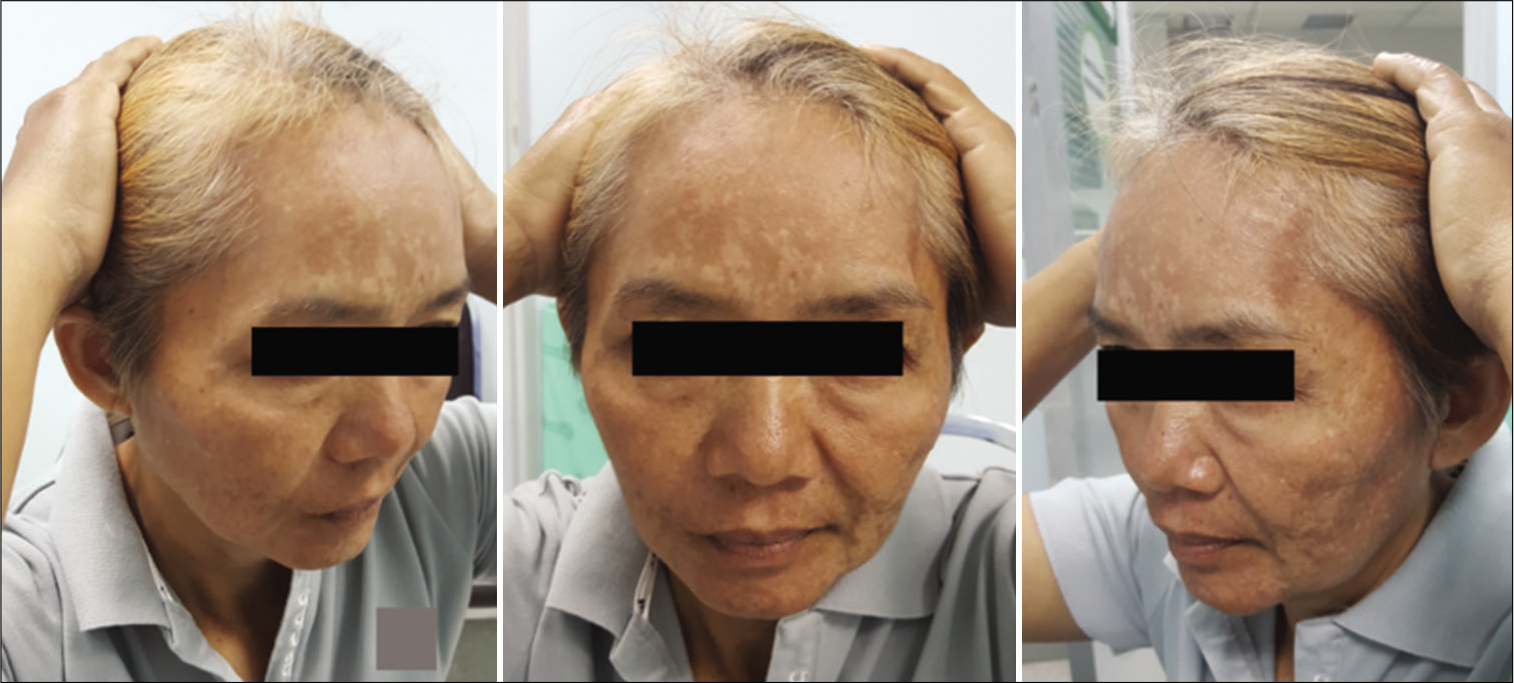
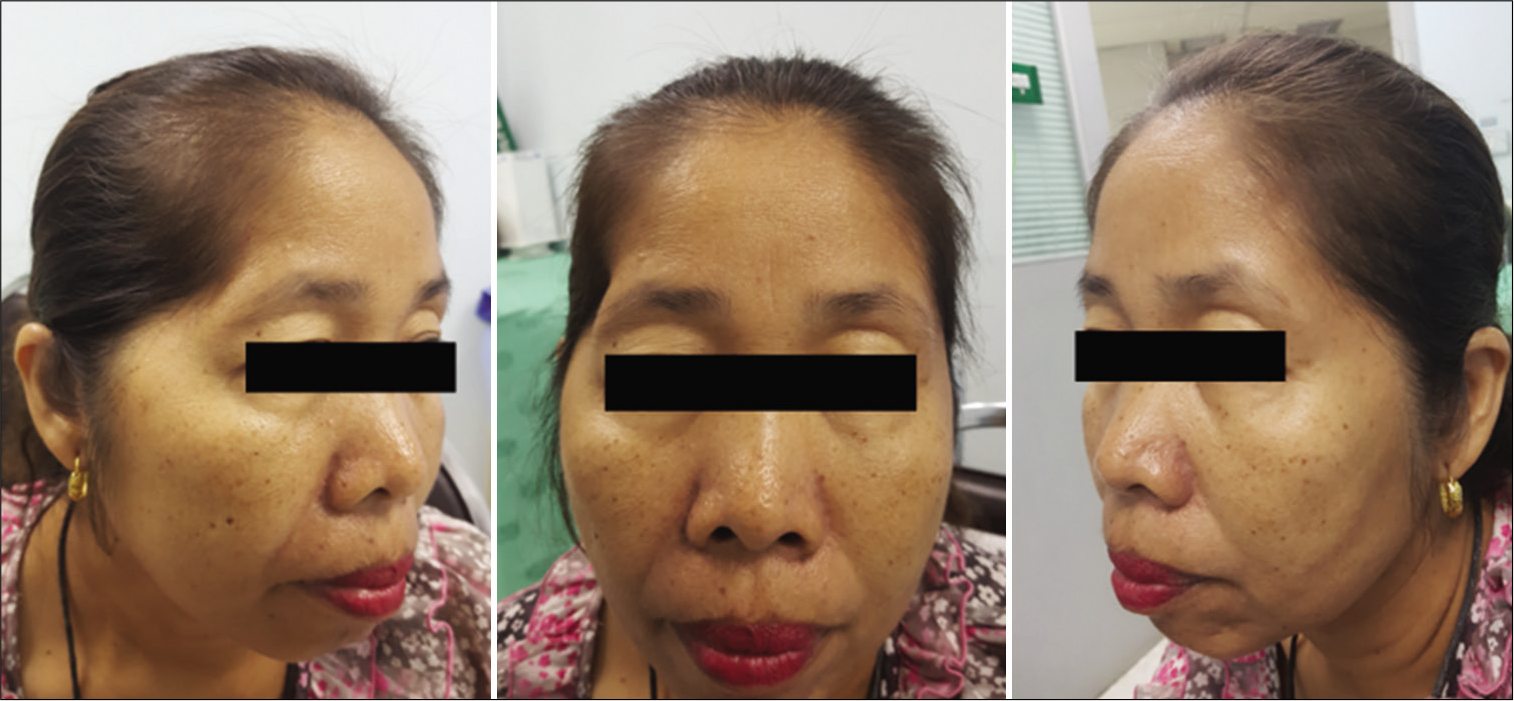
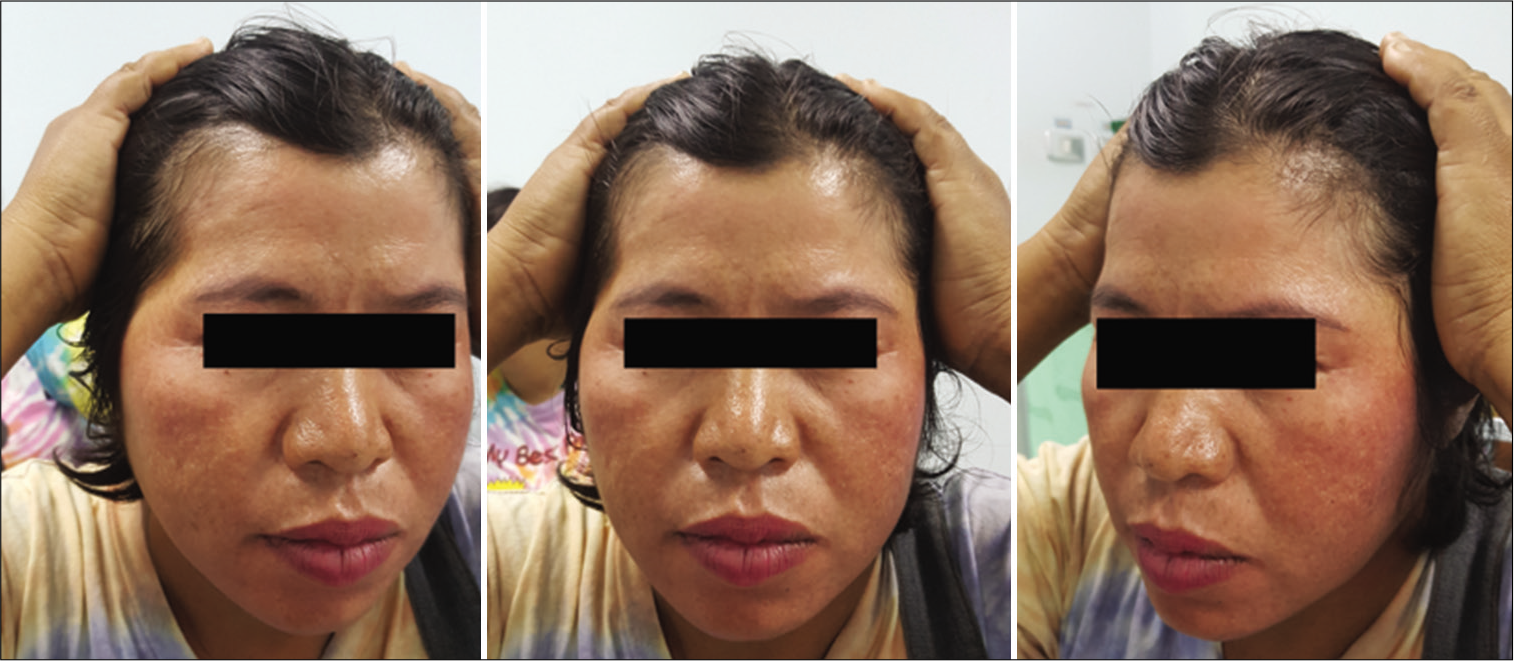
The function of the FTFN was assessed by the action of the frontalis muscle immediately or within 1 month after the operation and at the outpatient clinic. Postoperative temporal hollowing was evaluated 6–9 months after surgery. Degrees of temporal hollowing were classified as mild, moderate, and severe by comparison to the nonoperative side in the same patient. Temporal hollowing, which is only observed by closed inspection, was defined as mild. The temporal asymmetry detected at 1 and 2-m from the patient was defined as moderate and severe hollowing, respectively. Obvious hollowing was defined for moderate and severe.
Statistical analysis
Correlations between the postoperative frontalis paralysis, temporal hollowing, and surgical techniques were analyzed. Statistical analysis was performed with SPSS software version 22 for Windows. Study results for categorical measurements and univariate analysis are presented as a percentage (%). Chi-squared/Fisher exact test was used to compare the significance of the study parameters on the categorical scale between the two study groups. Fisher exact test was used when more than 20% of cells had expected frequencies <5. A P <0.05 was considered statistically significant.
RESULTS
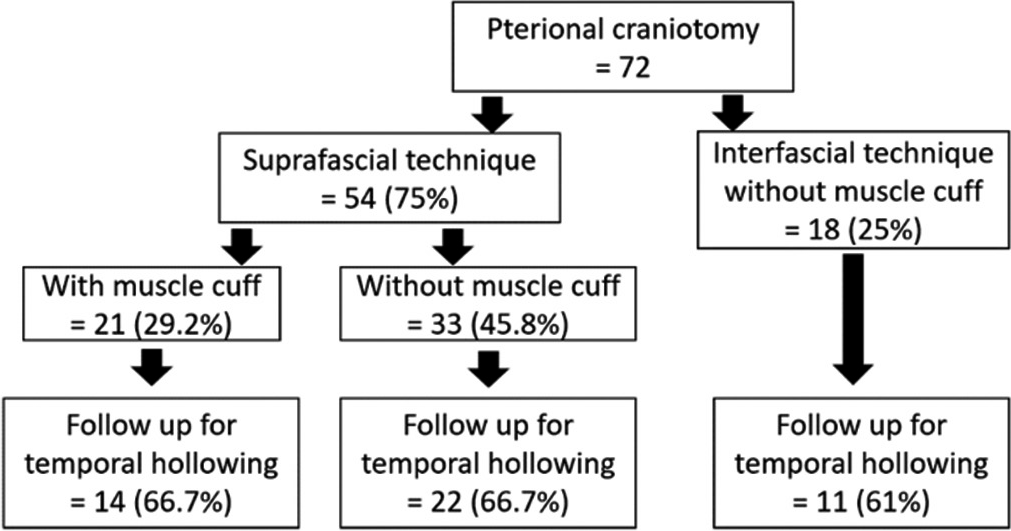
A total of 72 patients were included in this study. Fifty-four patients (75%) underwent pterional craniotomy using the suprafascial technique. In this group, the temporalis muscle cuff was left in 21 patients (29.2%) and not left in 33 patients (45.8%). The interfascial technique without muscle cuff was used in 18 patients (25%) [
Postoperative frontalis paralysis
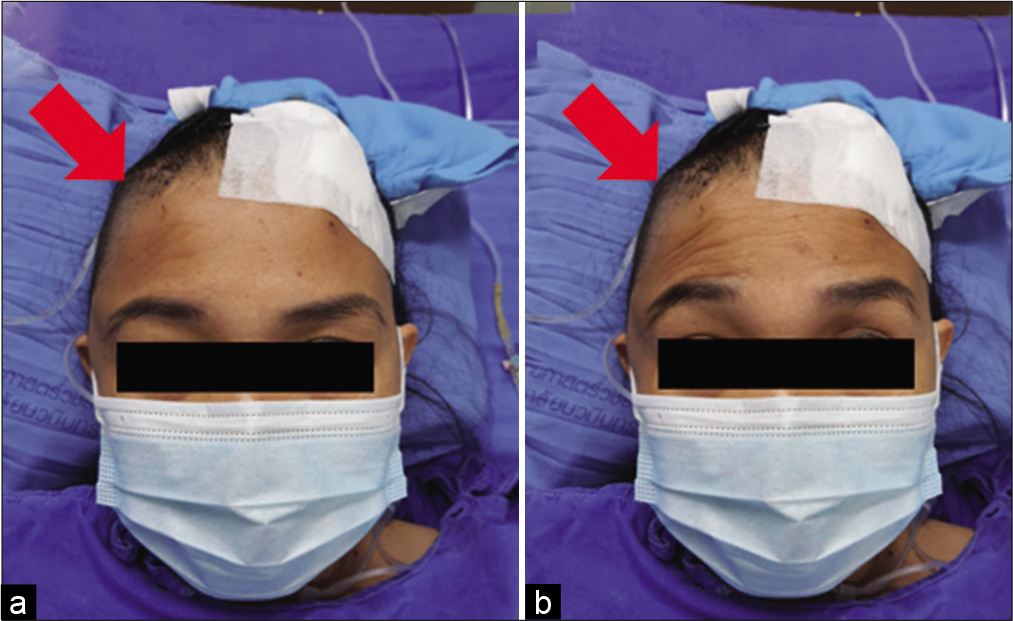
Ipsilateral frontalis muscles were evaluated in all patients immediately or within 1 month after the operation. In the suprafascial group, frontalis paralysis was not detected in 43 patients (79.6%) [
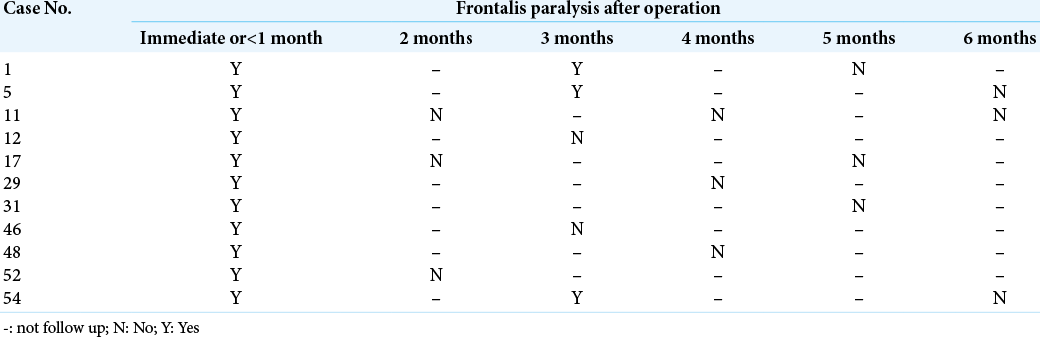
In the suprafascial group, all postoperative frontalis paralysis was transient. The possible times of recovery from paralysis ranged from 1 month to 6 months after surgery. The majority of the paralytic patients (7 in 11 cases; 63.6%) seem to improve within 4 months after the operation [
Postoperative temporal hollowing
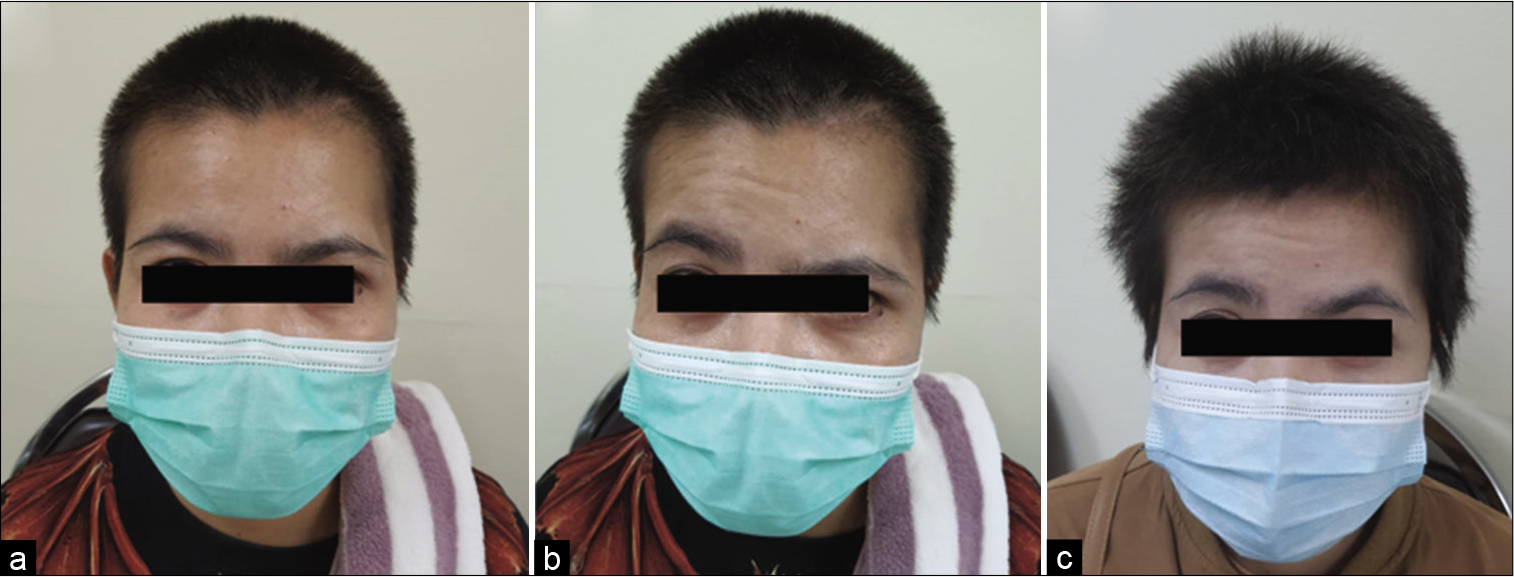
Among 54 patients who underwent the suprafascial dissection technique, 36 patients (66.7%) were also monitored for postoperative temporal hollowing at 6–9 months after the operation. In this group, 14 patients (66.7%) in the muscle cuff-on subgroup and 22 patients (66.7%) in the muscle cuff-off subgroup met the follow-up criteria [
Statistical difference was demonstrated between the suprafascial and interfascial groups (P = 0.03) [
DISCUSSION
Anatomy of the FTFN
The facial nerve exits the stylomastoid foramen, crosses the mandible at approximately 2.5 cm below the zygomatic arch, and enters the parotid gland where it divides into the frontal (temporal or frontotemporal), zygomatic, buccal, marginal mandibular, and cervical branches. The FTFN exits the parotid gland and runs anterosuperiorly to cross the zygomatic arch at approximately 2 cm from the tragus and gives three main rami: the auricularis (posterior rami), frontalis (middle rami, also known as the frontal branch), and orbicularis (anterior rami). The frontal (middle) rami innervate the frontalis muscle, which elevates the ipsilateral eyebrow.[
At the level of the zygomatic arch, the FTFN crosses in the fibro-fatty layer underneath the galea aponeurotica (also called temporoparietal fascia). The fibro-fatty layer, which is the fibrous adhesion of the galea, suprafascial (subgaleal) fat pad, loose areolar tissue and superficial temporal fascia, is located above the anteroinferior one-third of the temporalis muscle (3 cm posterior to the frontozygomatic process and 2.3 cm above the root of zygoma). This fibrous adhesion between the galea and superficial temporal fascia makes the separation of the two layers difficult and results in high risk of injury to the FTFN. Therefore, the elevation of the galea and skin from the superficial temporal fascia in this area should be avoided to minimize the risk to the FTFN [
Superior to the fibro-fatty layer, the FTFN runs just underneath the galea aponeurotica, courses superficially to the loose areolar tissue and suprafascial (subgaleal) fat pad, and runs anterosuperiorly to innervate the frontalis muscle. In this area, the plane between the galea and superficial temporal fascia is separated by loose areolar tissue without adhesion, which makes the elevation of the galea and the loose areolar tissue from the superficial temporal fascia easy with minimal risk of FTFN injury.[
Many tissue layers and cleavage planes exit beneath the FTFN. From the superficial to deep layer in the area superior to the fibro-fatty tissue, the loose areolar tissue, subgaleal (suprafascial) fat pad, superficial temporal fascia, interfascial (intrafascial) fat pad, deep temporal fascia, subfascial (deep) fat pad, and temporalis muscle are arranged subsequently above the skull. Many techniques for scalp flap elevation, such as subfascial, interfascial, and suprafascial (subgaleal) dissection, have been proposed to protect the FTFN [
The interfascial and subfascial dissection technique
To maximize the exposure of the pterional area, the two-layer technique of scalp flap creation was needed, but the risk of FTFN injury remained a concern. In 1987, Yasargil et al. first proposed the interfascial technique to protect the FTFN during pterional craniotomy.[
The suprafascial dissection technique
Although suprafascial (subgaleal) dissection of the skin flap results in a 30% incidence of ipsilateral frontalis nerve paralysis according to the technical article of Yasargil et al., no clinical data for this technique were provided.[
In the field of plastic and reconstructive surgery, suprafascial dissection was frequently used for access to the anterior and lateral facial skeleton. In 2008, Matic and Kim performed the prospective randomized controlled trial for postoperative temporal hollowing after bicoronal incision for facial bone surgery. The results were (1) the suprafascial dissection resulted in no postoperative frontalis paralysis and significantly less temporal hollowing (45%) than interfascial dissection (76% for dissection above the interfascial fat pad and 71% for dissection within the interfascial fat pad) at 6 months after surgery, and (2) decreased volumes of interfascial fat pad were negatively corelated with an increase in temporal hollowing severity.[
Postoperative transient frontalis paralysis
Both postoperative frontalis paralysis, which has an unclear incidence, and temporal hollowing are common complications after pterional craniotomy. In this study, the incidences of frontalis paralysis were 20.4% and 5.6% in suprafascial dissection and interfascial dissection, respectively. The incidence of postoperative frontalis paralysis tended to be higher with the suprafascial technique than interfascial technique, but no statistically significant difference was observed, which may be due to the small sample size. The natural history of frontalis paralysis was transient and spontaneously resolved within 6 months after the operation. No permanent frontalis paralysis was found after using both dissection techniques. No previous report of postoperative frontalis paralysis after pterional craniotomy was found. The incidence of frontalis paralysis in the suprafascial group of this study (20.4%) was higher than that reported by Matic and Kim[
With suprafascial and interfascial dissection, postoperative frontalis paralysis was transient. Therefore, the possible mechanism of paralysis is temporary nerve dysfunction (neurapraxia) caused by tissue manipulation during the dissection process and the retraction force of the scalp hooks. In this study, the incidence of frontalis paralysis in the suprafascial group (20.4%), which more than that in the interfascial group (5.6%), may be due to the dissection in the suprafascial plane, which is closer to the FTFN than dissection in the interfascial plane.
Postoperative temporal hollowing
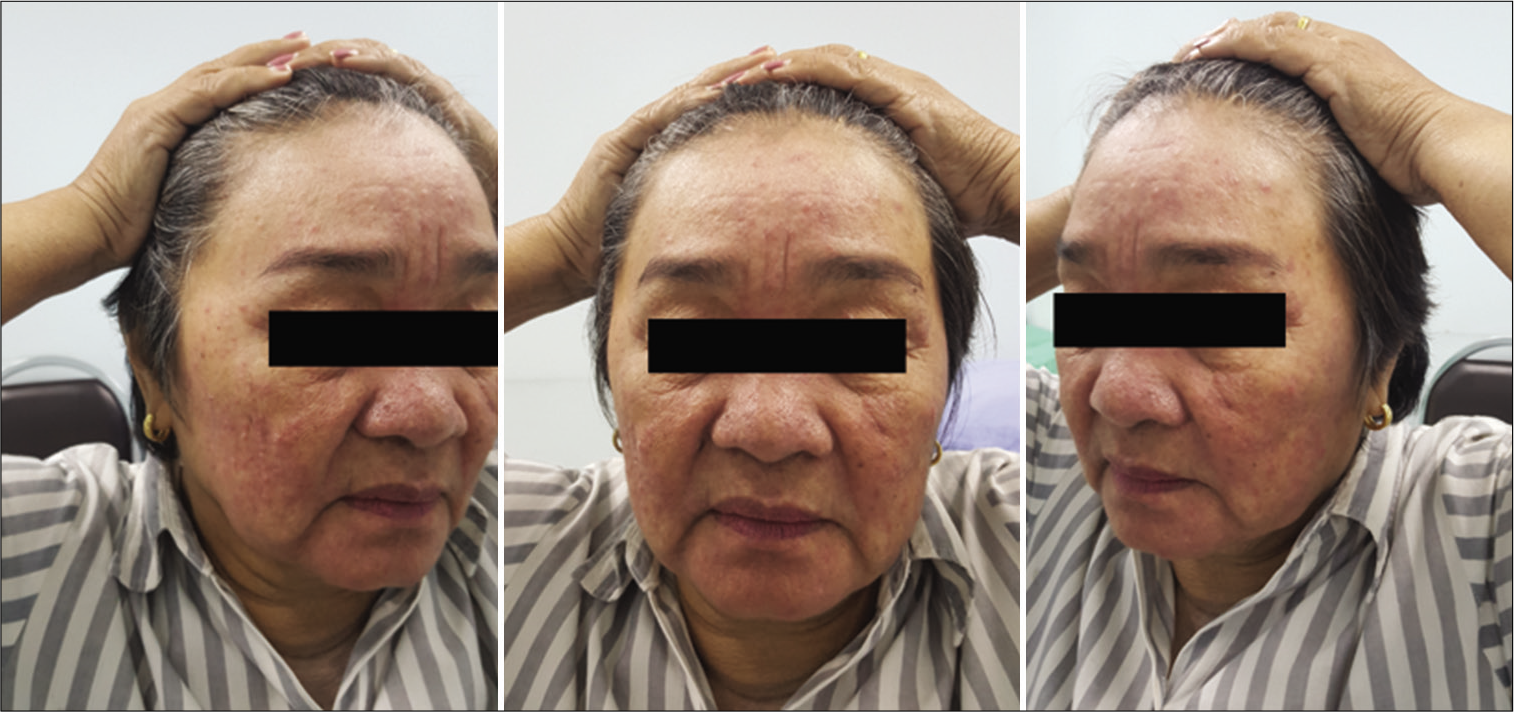
In this study, the incidence of obvious temporal hollowing was significantly high in the interfascial group (72.7%) and the suprafascial group with muscle cuff (64.3%), compared with the suprafascial group without muscle cuff (18.2%). The analysis was separated into two subgroups. In the first subgroup analysis of no muscle cuff-the cuff-off suprafascial group compared with the cuff-off interfascial group-the incidence of temporal hollowing was significantly high in the interfascial group. This indicates that interfascial dissection is the risk factor for temporal hollowing. In the second subgroup analysis of the suprafascial technique-retaining the muscle cuff (cuff-on) compared with not retaining the muscle cuff (cuff-off)-the incidence of temporal hollowing was significantly higher in the cuff-on group. This indicates that retaining the muscle cuff is the other risk factor for temporal hollowing, which corresponds to a previous report by Thiensri et al.[
In this study, suprafascial dissection without leaving the muscle cuff, which does not expose and cut the interfascial fat pad, caused an 18.2% incidence of temporal hollowing. This suggests that the atrophy of the temporalis muscle[
For the interfascial dissection technique for pterional craniotomy, previous reports demonstrated 62–78% incidence of moderate and severe temporal hollowing at 3–6 months after operation which is comparable to this study (72.7%).[
Gonçalves et al. performed systematic review and meta-analysis regarding the postoperative frontalis paralysis and temporal hollowing after pterional craniotomy with various modified techniques such as minipterional craniotomy, supraorbital craniotomy, lateral mini-orbitotomy, osteoplastic craniotomy, and subgaleal/myocutaneous dissection. Although these techniques seemed to minimize the postoperative complications, but reduced the size of craniotomy and exposure and required operative time. The suprafascial technique and the role of muscle cuff leaving were not included in this review.[
The indications for the interfascial dissection
Salas et al. discovered the fibro-fatty layer located just above the anteroinferior one-third of deep temporal fascia and proposed the appropriate indication for interfascial dissection. When exposure of the zygomatic bone or zygomatic arch is required (zygomatic arch osteotomy or orbitozygomatic osteotomy), interfascial dissection should be performed to protect the FTFN. When exposure of only the frontozygomatic process is required (frontal or pterional approach), interfascial dissection is not necessary, and the suprafascial technique can be performed.[
Limitations
Relatively high percentage of the transient frontalis paralysis (20.4%) and the incidence of postoperative temporal hollowing (18.2% in the cuff-off group) were the limitations of the suprafascial dissection technique compared to the interfascial and the one-layer (myocutaneous flap) technique respectively. The exposure of the zygomatic arch for orbitozygomatic osteotomy was another limitation of the suprafascial technique.[
The limitations of this study are its retrospective design, the small number of patients, and the relatively small percentage of patients (61–66.7%) who were followed long term to monitor temporal hollowing. Because most neurosurgeons in our hospital routinely use the one-layer submuscular dissection technique to perform the pterional craniotomy, the number of patients available for this study was small. Many patients in our tertiary center hospital were transferred from another distant hospital, which resulted in the high rate of patients lost to follow-up. Another limitation is that the esthetic outcomes from the patient’s opinion was not evaluated in this study.
CONCLUSION
The suprafascial dissection technique does not cause the permanent injury of the FTFN and results in a significantly lower incidence of postoperative temporal hollowing than the interfascial dissection, especially without leaving a temporalis muscle cuff.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ammirati M, Spallone A, Ma J, Cheatham M, Becker D. An anatomicosurgical study of the temporal branch of the facial nerve. Neurosurgery. 1993. 33: 1038-43
2. Baek RM, Heo CY, Lee SW. Temporal dissection technique that prevents temporal hollowing in coronal approach. J Craniofac Surg. 2009. 20: 748-51
3. Baucher G, Bernard F, Graillon T, Dufour H. Interfascial approach for pterional craniotomy: Technique and adjustments to prevent cosmetic complications. Acta Neurochir (Wien). 2019. 161: 2353-7
4. Campero A, Ajler P, Paíz M, Elizalde RL. Microsurgical anatomy of the interfascial vein. Its significance in the interfascial dissection of the pterional approach. Oper Neurosurg (Hagerstown). 2017. 13: 622-6
5. Chaddad-Neto F, Campos Filho JM, Dória-Netto HL, Faria MH, Ribas GC, Oliveira E. The pterional craniotomy: Tips and tricks. Arq Neuropsiquiatr. 2012. 70: 727-32
6. Coscarella E, Vishteh AG, Spetzler RF, Seoane E, Zabramski JM. Subfascial and submuscular methods of temporal muscle dissection and their relationship to the frontal branch of the facial nerve. Technical note. J Neurosurg. 2000. 92: 877-80
7. Davidge KM, van Furth WR, Agur A, Cusimano M. Naming the soft tissue layers of the temporoparietal region: Unifying anatomic terminology across surgical disciplines. Neurosurgery. 2010. 67: ons120-9
8. de Andrade FC, de Andrade FC, de Araujo Filho CM, Filho JC. Dysfunction of the temporalis muscle after pterional craniotomy for intracranial aneurysms. Comparative, prospective and randomized study of one flap versus two flaps dieresis. Arq Neuropsiquiatr. 1998. 56: 200-5
9. Gonçalves DB, Dos Santos MI, de Cristo Rojas Cabral L, Oliveira LM, da Silva Coutinho GC, Dutra BG. Esthetics outcomes in patients submitted to pterional craniotomy and its variants: A scoping review. Surg Neurol Int. 2021. 12: 461
10. Horimoto C, Toba T, Yamaga S, Tsujimura M. Subfascial temporalis dissection preserving the facial nerve in pterional craniotomy-technical note. Neurol Med Chir (Tokyo). 1992. 32: 36-37
11. Katsuno M, Tanikawa R, Miyazaki T, Ota N, Noda K, Izumi N. Anterior temporal approach and extraduraltemporopolar approach for the surgery of basilar bifurcation and posterior projecting carotid aneurysm. Jpn J Neurosurg. 2010. 19: 733-41
12. Kim E, Delashaw JB. Osteoplastic pterional craniotomy revisited. Neurosurgery. 2011. 68: 125-9
13. Kim S, Matic DB. The anatomy of temporal hollowing: The superficial temporal fat pad. J Craniofac Surg. 2005. 16: 760-3
14. Matic DB, Kim S. Temporal hollowing following coronal incision: A prospective, randomized, controlled trial. Plast Reconstr Surg. 2008. 121: 379e-85e
15. Matsukawa H, Tanikawa R, Kamiyama H, Tsuboi T, Noda K, Ota N. Localization in the interpeduncular cistern as risk factors for the thalamoperforators’ ischemia, poor outcome, and oculomotor nerve palsy in patients with complex unruptured basilar apex aneurysm treated with neck clipping. World Neurosurg. 2015. 84: 475-82
16. Park J, Hamm IS. Cortical osteotomy technique for mobilizing the temporal muscle in pterional craniotomies. Technical note. J Neurosurg. 2005. 102: 174-8
17. Poblete T, Jiang X, Komune N, Rhoton AL. Preservation of the nerves to the frontalis muscle during pterional craniotomy. J Neurosurg. 2015. 122: 1274-82
18. Salas E, Ziyal IM, Bejjani GK, Sekhar LN. Anatomy of the frontotemporal branch of the facial nerve and indications for interfascial dissection. Neurosurgery. 1998. 43: 563-9
19. Santiago GF, Terner J, Wolff A, Teixeira J, Brem H, Huang J. Post-neurosurgical temporal deformities: Various techniques for correction and associated complications. J Craniofac Surg. 2018. 29: 1723-9
20. Spetzler RF, Lee KS. Reconstruction of the temporalis muscle for the pterional craniotomy. Technical note. J Neurosurg. 1990. 73: 636-7
21. Spiriev T, Ebner FH, Hirt B, Shiozawa T, Gleiser C, Tatagiba M. Fronto-temporal branch of facial nerve within the interfascial fat pad: Is the interfascial dissection really safe?. Acta Neurochir (Wien). 2016. 158: 527-32
22. Spiriev T, Poulsgaard L, Fugleholm K. Techniques for preservation of the frontotemporal branch of facial nerve during orbitozygomatic approaches. J Neurol Surg B Skull Base. 2015. 76: 189-94
23. Takeuchi S, Tanikawa R, Tsuboi T, Noda K, Oda J, Miyata S. Superficial temporal artery to proximal posterior cerebral artery bypass through the anterior temporal approach. Surg Neurol Int. 2015. 6: 95
24. Thiensri T, Limpoka A, Burusapat C. Analysis of factors associated with temporal hollowing after pterional craniotomy. Indian J Plast Surg. 2020. 53: 71-82
25. Vaca EE, Purnell CA, Gosain AK, Alghoul MS. Postoperative temporal hollowing: Is there a surgical approach that prevents this complication? A systematic review and anatomic illustration. J Plast Reconstr Aesthet Surg. 2017. 70: 401-15
26. Yasargil MG, Reichman MV, Kubik S. Preservation of the frontotemporal branch of the facial nerve using the interfascial temporalis flap for pterional craniotomy. Technical article. J Neurosurg. 1987. 67: 463-6