- Department of Neurosurgery, University of Baghdad, College of Medicine, Baghdad, Iraq
- Department of Neurosurgery, University of Mustansiriyah, College of Medicine, Baghdad, Iraq,
- Department of Neurosurgery, Hannover Medical School, Hannover, Germany,
- Department of Neurosurgery, Neurosurgery Teaching Hospital, Baghdad, Iraq,
- Department of Neurosurgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States.
Correspondence Address:
Samer S. Hoz, Department of Neurosurgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States.
DOI:10.25259/SNI_597_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Zahraa M. Kareem1, Ahmed Muthana1, Sarah F. Hassan1, Fatimah Oday Ahmed2, Rania Thamir Hadi1, Hagar A. Algburi1, Oday Atallah3, Mustafa Ismail4, Samer S. Hoz5. Supraorbital artery: Anatomical variations and neurosurgical applications. 08-Sep-2023;14:318
How to cite this URL: Zahraa M. Kareem1, Ahmed Muthana1, Sarah F. Hassan1, Fatimah Oday Ahmed2, Rania Thamir Hadi1, Hagar A. Algburi1, Oday Atallah3, Mustafa Ismail4, Samer S. Hoz5. Supraorbital artery: Anatomical variations and neurosurgical applications. 08-Sep-2023;14:318. Available from: https://surgicalneurologyint.com/surgicalint-articles/12539/
Abstract
Background: The supraorbital artery (SOA) originates from the ophthalmic artery in a superomedial aspect of the orbit, exiting through the supraorbital groove to emerge onto the forehead. The SOA has important neurosurgical considerations regarding different approaches and bypasses. The SOA is poorly described in the standard anatomical textbooks. Therefore, we present this article to describe the anatomical variations of the SOA and their implications on the neurosurgical field.
Methods: We conducted a literature review in PubMed and Google Scholar databases to review the existing literature describing the SOA anatomy and its neurosurgical applications.
Results: While reading the available articles and original works regarding SOA, we identified 22 studies that discuss the SOA. We noticed the anatomical variations of the SOA in terms of origin, course, diameter, branches, depth, and distance in relation to the midline and vertical glabellar line. We also discussed certain applications of SOA and its importance in neurosurgical approaches, bypass, photoplethysmography, aneurysms, and reconstruction of cranial fossa defects.
Conclusion: The variable anatomy of the SOA has a paramount impact on performing different neurosurgical approaches. Therefore, cadaveric studies of the SOA are important to explore potential methods for the preservation of the artery in different neurosurgical applications.
Keywords: Anatomical variation, Neuroanatomy, Supraorbital artery
INTRODUCTION
The supraorbital artery (SOA) originates from the ophthalmic artery in a superomedial aspect of the orbit, exiting through the supraorbital groove to emerge onto the forehead. It ends with two terminal branches: superficial and deep. SOA supplies the skin of the forehead and the periosteum of the frontal bone, along with the superior rectus and levator palpebrae superioris muscles. Its terminal branches are anastomose with their contralateral counterparts, as well as the ipsilateral supratrochlear and superficial temporal arteries.[
The SOA has important neurosurgical considerations regarding different approaches and bypasses. This importance lies in its involvement in lesions such as aneurysms,[
The SOA is poorly described in the standard anatomical textbooks. The literature describing the SOA mostly fails to provide a precise, cumulative, or integrated description of the artery. An accurate definition of the SOA branches needs more attention to highlight the variance between SOA’s deep and superficial branches.[
For the purpose of drawing a complete picture of the anatomical disparities, we, hereby, describe the anatomical variations of the SOA and their implications on neurosurgical applications.
MATERIALS AND METHODS
A bibliographic search in Google Scholar and PubMed medical databases for studies on SOA and its clinical relevance was performed. The following search terms were used: “Supraorbital artery vascular anatomy” and “supraorbital artery neurosurgical applications.” Inclusion criteria were as follows, (i) English language and (ii) suitable methodology of the targeted data, while exclusion criteria were as follows, (i) non-English papers and (ii) questionable results. Results were categorized and selected appropriately. The data extraction includes surgical anatomy and neurosurgical applications of the SOA.
RESULTS
While reviewing the available articles and original works regarding SOA, considering the inclusion and exclusion criteria, we identified 22 articles that discuss the anatomical variations and neurosurgical applications of SOA. Certain parameters are used to describe the surgical anatomy of SOA, including origin, course, diameter, branches, depth, and distance in relation to the midline and vertical glabellar line. We also discussed certain applications of SOA and its importance in neurosurgical approaches, bypass, photoplethysmography, aneurysms, and reconstruction of cranial fossa defects.
DISCUSSION
SOA anatomy
SOA is one of the arteries that have considerable variations in anatomy. Certain parameters are implicated to discuss these variations, including origin, course, branches, diameter, depth, distance from midline, and its relationship with the vertical glabellar line.
SOA origin
The SOA stems from the ophthalmic artery, lying medially to the optic nerve. At the orbital rim, the ophthalmic artery bifurcates into superior and inferior orbitoglabellar arteries in 65% of cases, according to Tansatit et al. At this junction, the SOA emerges from the superior orbitoglabellar artery (37.5%) either after passing underneath the corrugator supercilii muscle near the supraorbital foramennotch or emerges separately from the ophthalmic artery through the supraorbital notch (25%) or foramen (10%) along the side of the supraorbital nerve.[
SOA course
The SOA courses 1–3 mm medially to the vertical midpupillary line. It travels lateral to the supratrochlear vasculature, close to the frontal bone. The SOA then either passes medially, where it pierces the corrugator supercilii muscle, or laterally where it has no connection with the muscle.[
SOA branches
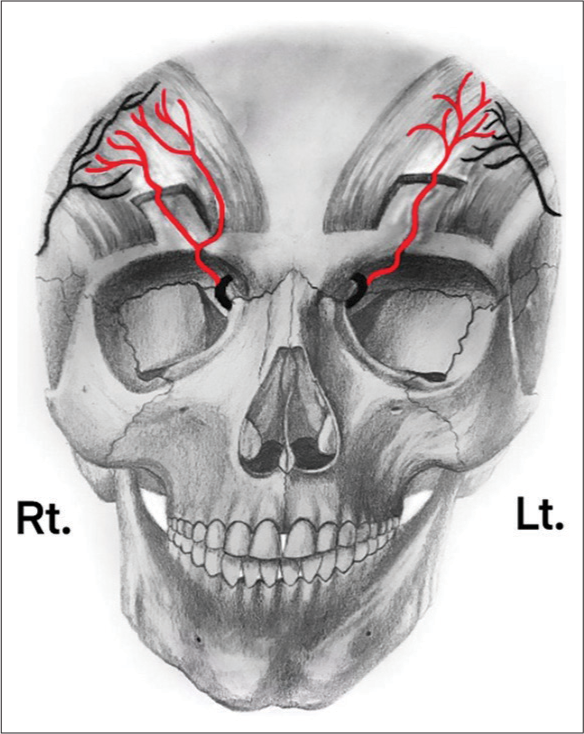
There are two possible configurations for the SOA. In the first, the SOA bifurcates forming superficial and deep branches once it exits the supraorbital foramen. In the second, its deep branch extends superficially and superolaterally from the supraorbital notch, piercing the frontalis muscle and forming the superficial branch [
Figure 1:
Anatomical variation of superficial and deep branches of SOA. On the right (Rt) hemi-forehead, SOA divides into superficial and a deep branches after it exits the supraorbital foramen. On the left (Lt) hemiforehead, deep branch of SOA extends superficially and superolaterally from the supraorbital notch; then, it pierces the frontalis muscle forming the superficial branch. SOA: Supraorbital artery.
Phumyoo et al. evaluated the SOA[
On the other hand, the deep branch is recognized in 100% of cases. In 60.5% of cadavers, it stems from the superior orbitoglabellar artery, and in cadavers 39.5% at the supraorbital notch. Typically, the SOA artery has a singular deep branch; nevertheless, Phumyoo et al. detected the presence of a double deep branch on the same side, as medial and lateral segments, in 34.2% of cadavers.[
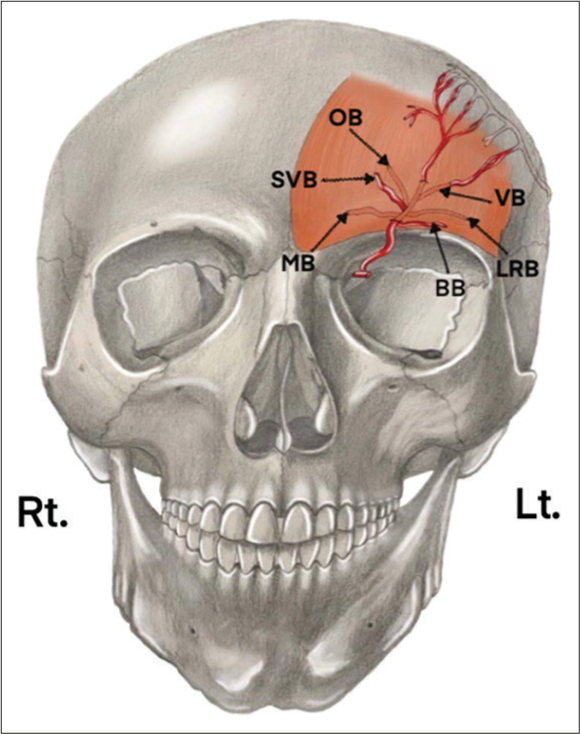
Kleintjes identified the terminal branches of the SOA, in order of frequency; deep branches include the vertical (100%), oblique, and lateral rim (91% each), and medial (44%) branches. Superficial branches include; superficial vertical (9%) and brow (5%) branches [
Figure 2:
The left (Lt) hemi-forehead view demonstrates the terminal branches of the supraorbital artery. This includes the superficial branches on the left hemi-forehead: brow branch (BB) and superficial vertical branch (SVB), as well as the deeper branches: medial branch (MB), oblique branch (OB), vertical branch (VB), and lateral rim branch (LRB).
Terminal branches of SOA deviate laterally at two-thirds of the eyebrow to anastomose with the superficial temporal artery frontal branch, confirming the existence of anastomosis between the two carotid systems.[
The incidence of SOA superficial branches is inconsistent, making it a poor candidate for consideration when planning skin flaps. On the other hand, the consistent existence of the SOA deep branches with their supplied areas marks them as important parameters for planning SOA-based flaps, taking into consideration varying and small contributions from the supratrochlear artery.[
Superficial branch
The branch progressively travels in a superficial plane around 20–40 mm beyond the supraorbital rim, and pierces the frontalis muscle to become superficial to this muscle until it reaches the region of mid-frontal depression.[
This branch first penetrates the corrugator supercilii muscle, followed by the frontalis muscles right underneath the midfrontal depression, 7.8 mm transversely from the medial canthus, and 11.5 mm vertically from the orbital rim [
Deep branch
The deep branch emerges from the SOA at the supraorbital rim, and passes about 16–42 mm beyond this point, traveling beneath the frontalis muscle, and on the pericranium contained by the subgaleal fascia.[
It, then, travels superolaterally, piercing the frontalis muscle and emerging on the other side of the muscle as the superficial branch.[
The medial, lateral rim and oblique branches are invariably deep to the periosteum. The vertical branch tends to branch into multiple tributaries shortly after its source, which is typically deep (periosteal). The oblique branch passes on the periosteum, then at the vertical line of the lateral orbital rim, where it enters the frontalis muscle anteriorly to anastomose with either the superficial temporal artery (STA) frontal branch or transverse frontal artery. Anastomosis typically takes place at the junction of the inferior and middle transverse thirds. However, it may occur at a higher transverse level, which gives the oblique branch an elongated course in the lateral forehead.[
SOA diameter
The average diameter of the SOA is 0.6 mm at the supraorbital rim for both deep and superficial branches, ranging from 0.5 to 1.4 mm at the horizontal mid-eyebrow level, which is somewhat larger compared to other levels.[
SOA depth
The mean distance between the SOA and the skin surface is around 3.54 mm, ranging from 2.2 to 6.0 mm.[
Distance of SOA from the midline
The mean distance of the SOA from the midline is 24.08 ± 5.5 mm with a range of 8.8–31.8 mm in males, while it is around 23.44 mm ranging from 14.0 to 32.2 mm in females.[
Relationship between SOA and vertical glabellar line
The mean distance between the midline and the vertical glabellar line at rest is around 5.55 mm for males and approximately 6.59 mm for females. The mean distance between the SOA and the ipsilateral vertical glabellar line is approximately 22.38 mm, ranging between 6.8 and 31.1 mm in males, and 20.73 mm, ranging from 6.2 to 28.8 mm in females.[
Neurosurgical applications of SOA
The SOA has important neurosurgical considerations; multiple approaches to the anterior cranial fossa, sellar and parasellar spaces, and orbit involve the artery.[
Related neurosurgical approaches
Eyebrow approach (transciliary or superciliary approach)
This approach is a modification to the standard pterional approach that is mostly related to the SOA and its anatomical variations. It delivers satisfactory visualization of the anterior fossa, the sellar, parasellar, and suprasellar territories. This approach can be used effectively to manage different lesions, such as aneurysms of the anterior circulation, and even skull base tumors, including pituitary adenomas, meningiomas, as well as craniopharyngiomas. During this approach, the SOA is the medial most boundaries to this approach. It is believed that deep lateral branches of the SOA can be affected during the transciliary approach.[
Superomedial transorbital approach
The superomedial transorbital approach can be employed with an extracranial-intracranial bypass to revascularize the intraorbital ophthalmic artery in cases of vascular occlusive disease of the artery. After the supraorbital foramen is identified, a “V-shaped” osteotomy is made to permit the release of the supraorbital nerve and SOA inferiorly, which are then displaced inferiorly in the direction of the periorbita.[
Transpalpebral approach
The SOA is an important landmark for the transpalpebral approach, along with the supraorbital nerve, supraorbital foramen, the frontal sinus, and the upper eyelid fold. This approach provides a surgical corridor to the anterior cranial fossa, parasellar region, orbital space, and frontal sinus. SOA is at risk of damage during this approach. As such, accurate localization of the supraorbital notch can decrease the risk of damaging the SOA, which, further, reduces its associated complications.[
Superficial temporal artery-SOA bypass
In cases of ocular ischemia associated with occlusive vascular diseases, it is believed that revascularization to the ophthalmic artery indirectly, through superficial temporal artery-SOA bypass, ameliorates visual activity in such circumstances. A study found that the use of superficial temporal artery-SOA bypass resulted in marked improvement of visual acuity in the affected eye and, surprisingly, in the contralateral eye as well. This may be attributed to enhanced blood flow in the ethmoidal collaterals.[
SOA photoplethysmography
An easy and rapid method that can be used safely to assess carotid artery occlusive disease, SOA plethysmographic curves can additionally be used to monitor bypass patency.[
Cirsoid aneurysms
Cirsoid aneurysms are uncommon arteriovenous fistulas of the scalp, and their cause is frequently inherited. The SOA can be one of the feeding arteries in scalp cirsoid aneurysms. These aneurysms are managed surgically; large vessels are separately ligated and divided, before raising the scalp flap, followed by careful dissection of the scalp from the cirsoid aneurysm, followed by ligating the feeding and draining vessels, including the SOA, and resecting the lesion together with underlying the pericranium. These steps are essential to obviate massive hemorrhage.[
Reconstruction flaps
Flaps used in the reconstruction of the skull base include; frontal and pericranial flaps,[
Frontal flap
In performing supraorbital craniotomies, the frontal flap is considered a rapid and safe material to reconstruct defects in the frontal sinus, anterior cranial fossa, and orbit.[
Pericranial flaps
These have been an invaluable resource for anterior cranial fossa reconstruction before the development of the nasoseptal flap. The flap can be designed according to its blood supply into anteriorly and laterally based on pericranial flaps. The anteriorly based pericranial flap is supplied primarily from the deep supraorbital and supratrochlear arteries.[
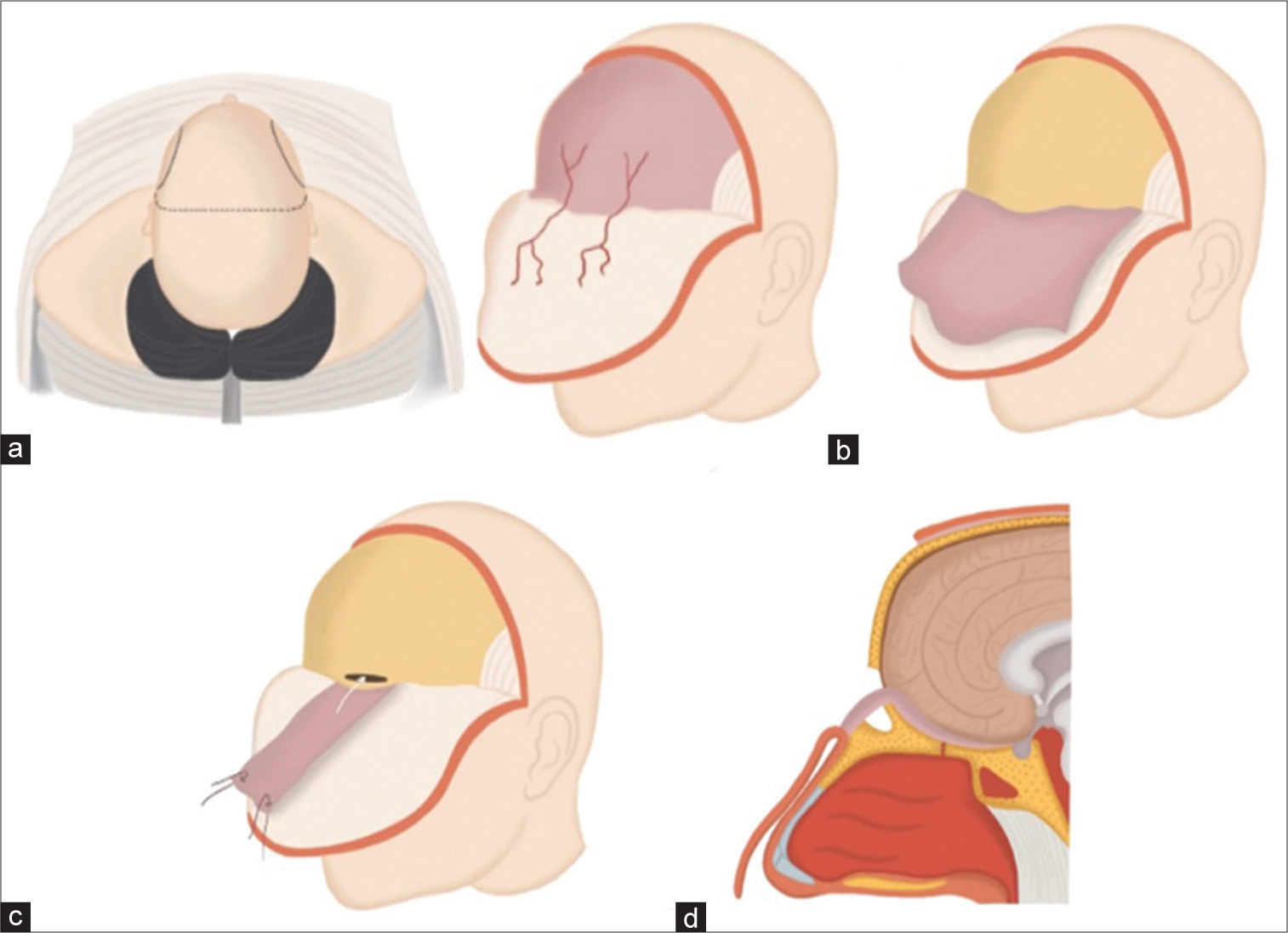
Figure 3:
(a) Illustration of a skull base reconstruction procedure employing a pericranial flap derived from the supraorbital artery. This procedure involves making a bicoronal skin incision and carefully peeling forward the skin and galea-frontalis layers. (b) This step in the process involves carefully separating the pericranium from the galea-frontalis layer. The pericranium, harvested as a flap, is a vital component for the subsequent stages of the skull base reconstruction. This flap provides the necessary coverage and support for the newly reconstructed area. (c) The image depicts a frontal craniotomy procedure, a crucial step in the skull base reconstruction process. This involves craniotomy of the frontal bone to access the underlying structures for repair and reconstruction. (d) This image illustrates the crucial step of introducing the pericranial flap into the frontal cranial cavity. The flap is skillfully maneuvered to reconstruct the defect in the anterior cranial fossa, thereby restoring the anatomical integrity of the skull base.
Temporofrontal fascial flap
Such a flap can penetrate the temporal muscle for the purpose of reconstructing the anterior and temporal skull base and dura. The arterial anatomical distribution of the t emporofrontal fascia flap is the connective arteries between the supraorbital and middle temporal arteries near the temporal line. The SOA is included in the periosteal part of the temporofrontal fascia flap.[
Reconstruction of skin defects
Flaps based on the SOA used in the reconstruction of skin defects include the island flaps.[
In a nutshell, appreciating the anatomy and variations of SOA is of great help in different surgical applications, enriching the surgeon’s armamentarium during surgery as an additive source of blood supply when other collaterals are insufficient in performing reconstruction flaps for skull base or skin defects, and the importance of its preservation during transcranial approaches to avoid creating a hematoma.
CONCLUSION
The variable anatomy of the SOA has a paramount impact on performing different neurosurgical approaches and bypasses. In addition, it constitutes an important secondary source of blood supply for skull base reconstruction and skin flaps when the primary sources are compromised. Therefore, cadaveric studies of the SOA as it pertains to new and updated approaches are essential to explore potential methods for the preservation of the artery in different neurosurgical applications.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Carstens MH, Greco RJ, Hurwitz DJ, Tolhurst DE. Clinical applications of the subgaleal fascia. Plast Reconstr Surg. 1991. 87: 615-26
2. Cavalcanti DD, García-González U, Agrawal A, Crawford NR, Tavares PL, Spetzler RF. Quantitative anatomic study of the transciliary supraorbital approach: Benefits of additional orbital osteotomy?. Neurosurgery. 2010. 66: 205-10
3. Cong LY, Phothong W, Lee SH, Wanitphakdeedecha R, Koh I, Tansatit T. Topographic analysis of the supratrochlear artery and the supraorbital artery: Implication for improving the safety of forehead augmentation. Plast Reconstr Surg. 2017. 139: 620e-7
4. Cotofana S, Lachman N. Arteries of the face and their relevance for minimally invasive facial procedures: An anatomical review. Plast Reconstr Surg. 2019. 143: 416-26
5. Delashaw JB, Jane JA, Kassell NF, Luce C. Supraorbital craniotomy by fracture of the anterior orbital roof. J Neurosurg. 1993. 79: 615-8
6. Dzhindzhikhadze RS, Dreval ON, Lazarev VA, Polyakov AV. Transpalpebral approach in skull base surgery: How I do it. Acta Neurochir (Wien). 2019. 161: 133-7
7. El Shazly AA, Saoud KM. Results of surgical excision of cirsoid aneurysm of the scalp without preoperative interventions. Asian J Neurosurg. 2012. 7: 191
8. Erdogmus S, Govsa F. Anatomy of the supraorbital region and the evaluation of it for the reconstruction of facial defects. J Craniofac Surg. 2007. 18: 104-12
9. Friedlander LD, Barrow DL, BaKay RA. Microsurgical revascularization of the ophthalmic artery. Skull Base Surg. 1995. 5: 191-8
10. Gevorgyan A, Yaghjyan GV, Shamakhyan HV, Danielyan AM, Sahakyan AB. Supraorbital artery myocutaneous island flap for forehead defect reconstruction. J Craniofac Surg. 2008. 19: 513-6
11. Hammad OY, Ali AK, Ezzat WA, Elbahy K, El Hakim AH. Mini craniotomy for anterior skull base lesions. Mortality. 2006. 1: 13
12. Herreras JI, Mediavilla JC, García MJ, Camarero SR. Carotid endarterectomy with Javid bypass and supraorbital photoplethysmography. Rev Esp Anestesiol Reanim. 1991. 38: 44-7
13. Kalani MY, Spetzler RF, Wanebo JE. Keyhole supraorbital craniotomy for aneurysm clipping in the setting of bypass for moyamoya disease. World Neurosurg. 2016. 94: 442-6
14. Katsuno M, Uchida K, Matsuno A. A temporofrontal fascia flap that penetrated temporal muscle for the reconstruction of an anterior skull base bone and Dura: A technical case report. Br J Neurosurg. 2019. 33: 272-4
15. Kleintjes WG. Forehead anatomy: Arterial variations and venous link of the midline forehead flap. J Plast Reconstr Aesthet Surg. 2007. 60: 593-606
16. Kuroda S, Houkin K, Ishikawa T, Nakayama N, Iwasaki Y. Novel bypass surgery for moyamoya disease using pericranial flap: Its impacts on cerebral hemodynamics and long-term outcome. Neurosurgery. 2010. 66: 1093-101
17. Phumyoo T, Jiirasutat N, Jitaree B, Rungsawang C, Pratoomthai B, Tansatit T. Localization and topography of the arteries on the middle forehead region for eluding complications following forehead augmentation: Conventional cadaveric dissection and ultrasonography investigation. J Craniofac Surg. 2020. 31: 2029-35
18. Rubio RR, Vigo V, Gandhi S, Tabani H, Meybodi AT, Winkler EA. An anatomical feasibility study for revascularization of the ophthalmic artery. Part II: Intraorbital segment. World Neurosurg. 2020. 133: 401-8
19. Safavi-Abbasi S, Komune N, Archer JB, Sun H, Theodore N, James J. Surgical anatomy and utility of pedicled vascularized tissue flaps for multilayered repair of skull base defects. J Neurosurg. 2016. 125: 419-30
20. Schwenn OK, Wüstenberg EG, Konerding MA, Hattenbach LO. Experimental percutaneous cannulation of the supraorbital arteries: Implication for future therapy. Invest Ophthalmol Vis Sci. 2005. 46: 1557-60
21. Tansatit T, Phumyoo T, Jitaree B, Sahraoui YM, Lee JH. Anatomical and ultrasound-based injections for sunken upper eyelid correction. J Cosmet Dermatol. 2020. 19: 346-52
22. Yoshioka N, Rhoton AL. Vascular anatomy of the anteriorly based pericranial flap. Neurosurgery. 2005. 57: 11-6