- Department of Neurosurgery, Neurosurgery Teaching Hospital, Baghdad, Iraq,
- Department of Neurosurgery, King Fahad Hospital of the University, Imam Abdulrahman Alfaisal University, Dammam, Saudi Arabia,
- Department of Neurosurgery, Strong Memorial Hospital University of Rochester, Rochester, New York, United States.
Correspondence Address:
Zahraa F. Al-Sharshahi
Department of Neurosurgery, Neurosurgery Teaching Hospital, Baghdad, Iraq,
DOI:10.25259/SNI_219_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hoz SS1, AL-Jehani H2, Al-Sharshahi ZF1, Matti WE1, Al-Dhahir MA3, Kadhum HJ1. Supratentorial brain tumors: Are there indications for urgent resection?. Surg Neurol Int 26-Apr-2021;12:194
How to cite this URL: Hoz SS1, AL-Jehani H2, Al-Sharshahi ZF1, Matti WE1, Al-Dhahir MA3, Kadhum HJ1. Supratentorial brain tumors: Are there indications for urgent resection?. Surg Neurol Int 26-Apr-2021;12:194. Available from: https://surgicalneurologyint.com/surgicalint-articles/10749/
Sir,
Supratentorial brain tumors (STBTs) usually manifest as progressive neurological symptoms and surgical management is typically planned in an elective or semi-elective setting.[
The question is, what are the circumstances, if any, that require an “urgent” resection of the STBT? In other words, what are the scenarios that point to the tumor mass itself as the main culprit in the deterioration at hand?
For the purposes of our discussion, we will arbitrarily use the term “urgent” to apply to tumor resection surgery performed within 24–48 h of the initial assessment. With this definition in mind, we retrospectively analyzed the data of all STBT cases managed at our institution over the past 6 years (2014–2020) to identify patients who underwent “urgent” resection of the tumor and the factors that contributed to that decision at the time. These parameters are not intended as “urgent” tumor resection criteria, but rather are used to provide insights into potential scenarios that may require “urgent” removal and, most importantly, to identify existing knowledge gaps in the field and to guide research endeavors.
A total of 58 patients were included. Our analysis showed that all patients who underwent “urgent” tumor resection were brought to the emergency department with an altered level of consciousness with an average Glasgow Coma Scale (GCS) of 9, range 7–11. In addition, 40% of them (n = 23) had hemiparesis or hemiplegia. In 95% of cases (n = 55), computed tomography (CT) scans revealed a significant midline shift, with a mean of 16 mm and a range of 12–20 mm. The average size of the resected tumors was 5.5 cm (range 4.4–6.6cm). In all subjects, the decision to operate followed a trial of failed medical management using dexamethasone and hyperosmolar therapy for a minimum of 6 h.[
Metastatic lesions were the most common tumor type, present in 45% (n = 26), followed by meningioma (25% n = 12), glioblastoma (20%, n = 11), and others 10% (n = 9). More lesions (57.9%, n = 34) were in the right hemisphere and tumor-related hemorrhage was present in 11% of cases (n = 6). The epicenter of the lesion was located in the temporal lobe in 53% of patients (n = 31). Other locations included the parietal area (25%, n = 15), frontal (17.2%, n = 10), and occipital (3.4%, n = 2). The average postoperative GCS was 14–15, and motor function improved in 87% (n = 20/23) of the cases.
The above figures suggest that in very particular circumstances, a set of factors may function cumulatively to indicate “urgent” resection. A representative scenario is a patient who is brought to the emergency department with acute and rapidly progressive neurological deterioration whose CT scan shows a large supratentorial tumor mass with significant midline shift and he/she fails to improve or continues to deteriorate despite attending to potential medical causes and intracranial pressure lowering therapy [
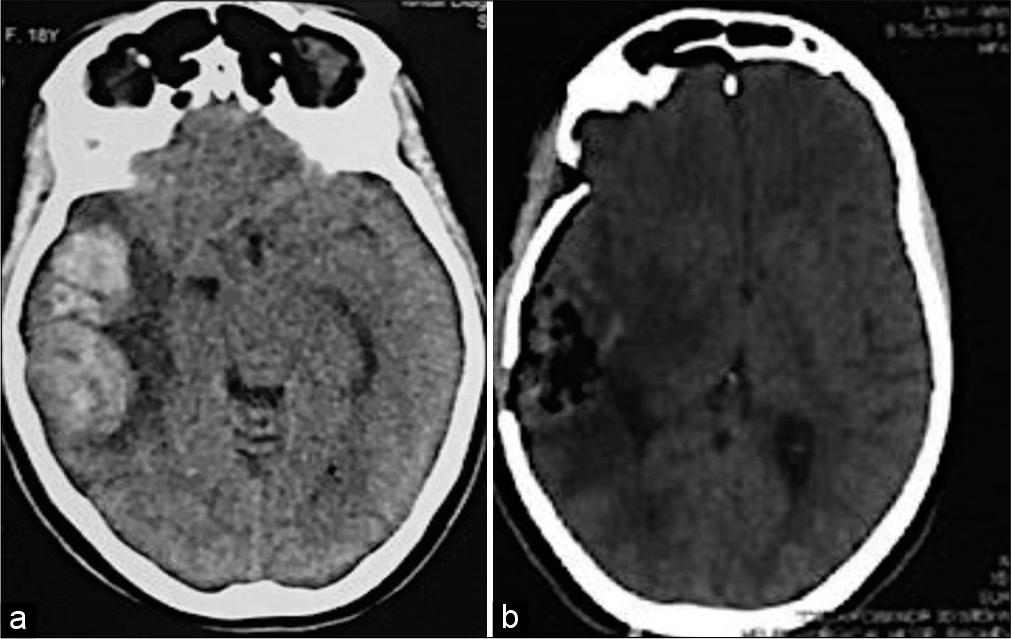
Figure 1:
Cranial computed tomography (CT) scan images (noncontrast, axial sections) of an 18-year-old female who was brought to the emergency department with a Glasgow Coma Scale (GCS) of 11 and a left-sided hemiparesis. Having excluded potential differentials, the patient was initially treated with dexamethasone and mannitol for 6 h, but continued to deteriorate. She underwent urgent tumor resection. Postoperatively, her postoperative and discharge GCS were 13 and 15, respectively, with improved weakness. (a) A right-sided, large, temporoparietal, ill-defined mass. Significant mass effects in the form of ipsilateral lateral ventricular compression and significant midline shift of >1 cm are seen. (b) Postoperative CT scan showing complete resection of the tumor with resolution of midline shift. Histopathological analysis showed that the tumor is a glioblastoma.
Figure 2:
Cranial computed tomography (CT) scan images (noncontrast, axial sections) of a 34-year-old gentleman who was admitted to the emergency department with a Glasgow Coma Scale (GCS) of 8, on a background history of headaches over the preceding 2 weeks. Having excluded potential differentials, the patient was initially treated with dexamethasone and mannitol for 7 h, but failed to improve. He underwent urgent tumor resection. Postoperatively, his postoperative and discharge GCS were 14 and 15, respectively, with improved weakness. (a) A left-sided large parietal, well-defined mass. Significant mass effects in the form of ipsilateral lateral ventricular compression and significant midline shift of >1 cm are seen. (b) Postoperative CT scan showing complete resection of the mass of the tumor with obvious improvement of midline shift. Histopathological analysis showed that the tumor is a meningioma.
This particular scenario poses four key questions: (1) is this patient a candidate for an “urgent” tumor resection? (2) How predictive are the factors listed above? (3) What is the cause of the acute deterioration? Specifically, is the current deterioration related to intralesional bleeding or is it a late manifestation of a large tumor? (4) Where do we draw the line for “medical treatment failure?”
To unveil these issues, the next steps would be (1) establish a management algorithm for excluding or managing the above-mentioned differentials. (2) Ordain recommendations for the maximum dose and length of steroid and hyperosmolar therapy in the way of defining the exact meaning and timing of medical treatment failure. (3) Conduct multicenter, retrospective, longitudinal studies which evaluate STBT cases operated “urgently” to identify predictive factors and analyze the need for decision-making criteria. (4) Validate the results of retrospective studies through large-scale clinical trials.
Responding to these questions could serve as a step forward in improving the evidence-based neurosurgical decision-making process. Having established requirements for “urgent” resection of STBTs will help neurosurgeons to be better equipped in managing neuro-oncology patients, and most importantly improve safety outcomes for this critical patient cohort, thus reducing possible delay in surgery and the associated potential repercussions.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abdulbaki A, Kanaan I. The impact of surgical timing on visual outcome in pituitary apoplexy: Literature review and case illustration. Surg Neurol Int. 2017. 8: 16
2. Arvold ND, Armstrong TS, Warren KE, Chang SM, DeAngelis LM, Blakeley J. Corticosteroid use endpoints in neuro-oncology: Response assessment in neuro-oncology working group. Neuro Oncol. 2018. 20: 897-906
3. Halstead MR, Geocadin RG. The medical management of cerebral edema: Past, present, and future therapies. Neurotherapeutics. 2019. 16: 1133-48
4. Jessurun CA, Hulsbergen AF, Cho LD, Aglio LS, Nandoe Tewarie RD, Broekman ML. Evidence-based dexamethasone dosing in malignant brain tumors: What do we really know?. J Neurooncol. 2019. 144: 249-64
5. Onitilo AA, Kio E, Doi SA. Tumor-related hyponatremia. Clin Med Res. 2007. 5: 228-37
6. Peng Y, Liu X, Wang A, Han R. The effect of mannitol on intraoperative brain relaxation in patients undergoing supratentorial tumor surgery: Study protocol for a randomized controlled trial. Trials. 2014. 15: 165
7. Snyder H, Robinson K, Shah D, Brennan R, Handrigan M. Signs and symptoms of patients with brain tumors presenting to the emergency department. J Emerg Med. 1993. 11: 253-8
8. Yaşargil MG, Abdulrauf SI. Surgery of intraventricular tumors. Neurosurgery. 2008. 62: 1029-40