- UT Health San Antonio Long School of Medicine, San Antonio, Texas, United States
- Department of Neurosurgery, Augusta University, Georgia, United States
- Department of Neurosurgery, UT Health San Antonio Long School of Medicine, San Antonio, Texas, United States
- Department of Radiation Oncology, University of Arkansas for Medical Sciences, Little Rock, Arkansas, United States
- Department of Hematology and Medical Oncology, UT Health San Antonio Long School of Medicine, San Antonio, Texas, United States,
- Department of Pathology, UT Health San Antonio Long School of Medicine, San Antonio, Texas, United States.
Correspondence Address:
Jacob A. Bethel, UT Health San Antonio Long School of Medicine, San Antonio, Texas, United States.
DOI:10.25259/SNI_984_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jacob A. Bethel1, Kenneth M. James2, Samon G. Tavakoli3, Richard L. Crownover4, Andrew J. Brenner5, Alexander M. Papanastassiou3, Andrea R. Gilbert6. Supratentorial ependymoma, zinc finger translocation-associated fusion positive, with extensive synaptophysin immunoreactivity arising from malignant transformation of clear cell ependymoma: A case report. 22-Apr-2022;13:168
How to cite this URL: Jacob A. Bethel1, Kenneth M. James2, Samon G. Tavakoli3, Richard L. Crownover4, Andrew J. Brenner5, Alexander M. Papanastassiou3, Andrea R. Gilbert6. Supratentorial ependymoma, zinc finger translocation-associated fusion positive, with extensive synaptophysin immunoreactivity arising from malignant transformation of clear cell ependymoma: A case report. 22-Apr-2022;13:168. Available from: https://surgicalneurologyint.com/surgicalint-articles/11547/
Abstract
Background: We describe a case of a supratentorial ependymoma, zinc finger translocation-associated (ZFTA) fusion positive with extensive synaptophysin immunoreactivity arising from malignant transformation of an ependymoma with clear cell features in a patient with long-term follow-up.
Case Description: A 55-year-old woman presented with seizures and ataxia 15 years after an initial resection of a clear cell ependymoma, Grade 2. Imaging demonstrated an enhancing right paracentral mass and the patient underwent biopsy and resection. Microscopic analysis showed regions of the tumor with morphological and immunohistochemical features typical of ependymoma, including perivascular pseudorosettes and focal dot- like epithelial membrane antigen positivity, as well as high-grade features. In addition, the neoplasm contained large nodular regions of clear cells exhibiting extensive synaptophysin immunoreactivity, suggestive of neural differentiation, and only focally positive immunoreactivity for glial markers. Electron microscopy showed poorly formed and ill-defined junctional complexes, but no cilia, microvilli, or dense granules were seen. Molecular profiling revealed the presence of a fusion between ZFTA (previously known as C11orf95) and RELA fusion.
Conclusion: We report a case of extensive synaptophysin immunoreactivity in a ZFTA-RELA fusion-positive ependymoma that had undergone malignant transformation from a clear cell ependymoma and has long-term follow-up, contributing to the assessment of prognostic significance of synaptophysin immunoreactivity in supratentorial ependymoma, ZFTA fusion positive.
Keywords: Anaplastic ependymoma, C11orf95, Clear cell ependymoma, Neuronal differentiation, RELA fusion, Zinc finger translocation associated
INTRODUCTION
Ependymomas are primary tumors of the central nervous system (CNS) that may occur at any site along the neuraxis. Microscopically, ependymomas are classically characterized by perivascular pseudorosettes, ependymal rosettes, glial fibrillary acidic protein (GFAP) reactivity, areas of fibrillary, and paranuclear dot and ring positivity for epithelial membrane antigen (EMA) immunohistochemical stain. Clear cell, papillary, and tanycytic ependymomas were once codified as distinct variants in previous editions of the World Health Organization (WHO) classification system,[
The fifth edition of the WHO classification of CNS tumors divides ependymomas into prognostically relevant groups based on a combination of anatomic location (i.e., supratentorial, posterior fossa, and spinal compartments) and molecular features.[
Supratentorial ependymoma, ZFTA fusion positive was originally codified in the revised fourth edition of the WHO classification system as ependymoma , RELA fusion positive. The discovery that ZFTA, previously known as C11orf95, may pair with fusion partners other than RELA to produce the same histomolecular neoplasm[
Intrachromosomal fusion between the RELA and ZFTA (previously known as C11orf95) forms an oncogenic driver of supratentorial ependymomas that are thought to be present in up to 70% of pediatric supratentorial ependymomas.[
CASE REPORT
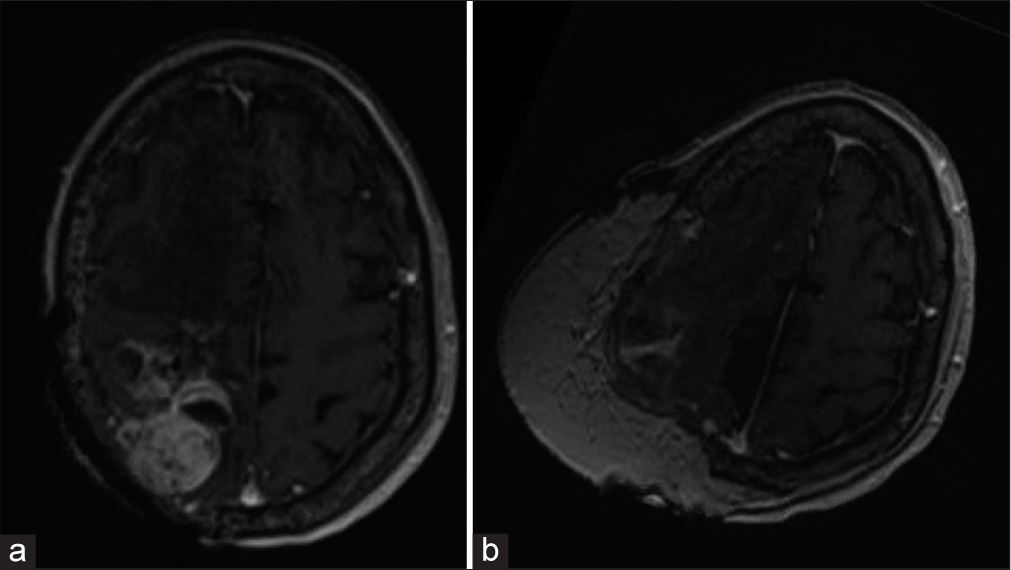
A 55-year-old woman with a history of a right frontoparietal clear cell ependymoma treated with surgery, adjuvant radiation therapy, and infusional chemotherapy 12 years before presented with symptomatic recurrence of a right frontoparietal mass. The specimen obtained from the initial resection [
Twelve years later, the patient developed breakthrough seizures and progressive left-sided weakness. Follow-up magnetic resonance imaging (MRI) of the brain identified an enhancing lobulated cortical lesion within her right frontoparietal lobes, in the same location as her original mass that was compressing the primary motor and sensory cortices and extending into the dura [
Figure 2:
Axial T1-weighted imaging post contrast demonstrating (a) focal post-surgical changes from resection twelve years prior and an enhancing cortical lesion within the right parietal lobe concerning for recurrence. (b) immediate post-surgical changes of the right frontoparietal craniotomy for first recurrence with peripheral residual enhancement in resection cavity and mild enhancement in the superior aspect of the frontal lobe.
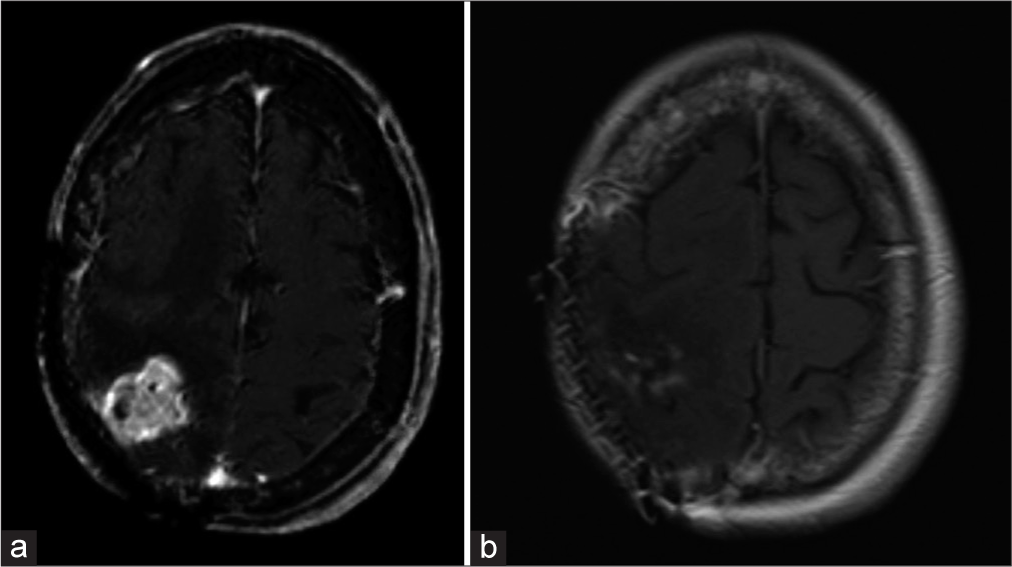
Fifteen years after her original presentation and 3 years after the treatment of her first recurrence, she presented with ataxia and left-sided hemiparesis severe enough that she did not have functional use of the left upper or lower extremity. In the same region as her prior tumors, MRI demonstrated a new right posterior parietal convexity, heterogeneously enhancing mass lesion arising along the superior aspect of the resection cavity within the right perirolandic cortex [
Figure 4:
(a) Second recurrence seventeen months after second resection with interval increased size of right posterior parietal convexity heterogeneously enhancing mass lesion measuring 3.7 x 4.6 x 2.9 cm with additional minimal adjacent nodular enhancement seen within the right superior frontal sulci/gyri and pachymeninges with an increase in associated vasogenic edema (b) Immediate post-surgical changes of right posterior parietal convexity mass resection for second recurrence with irregular brain parenchymal enhancement about the resection site and parietal scalp free flap reconstruction.
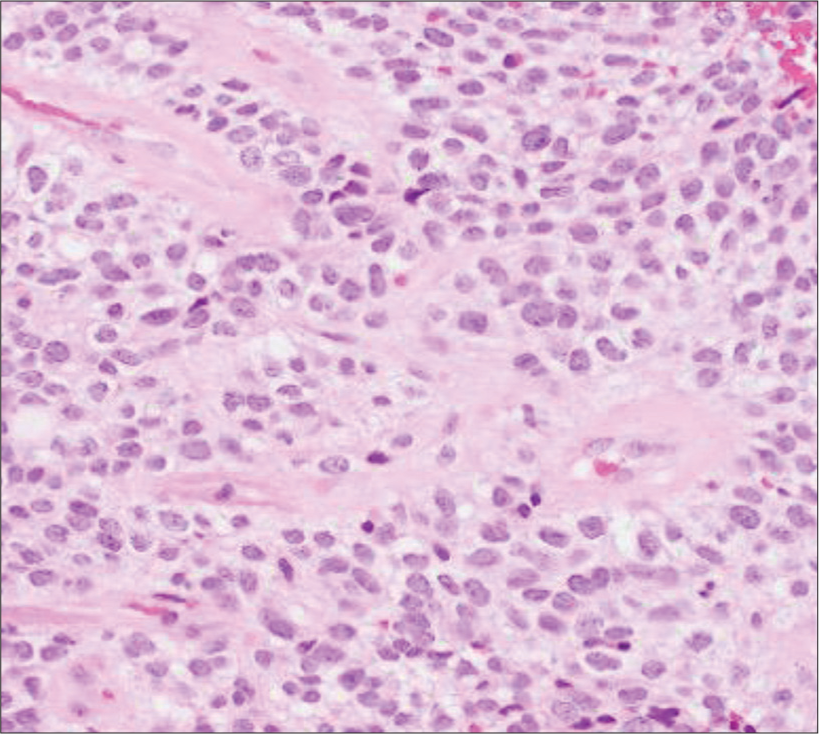
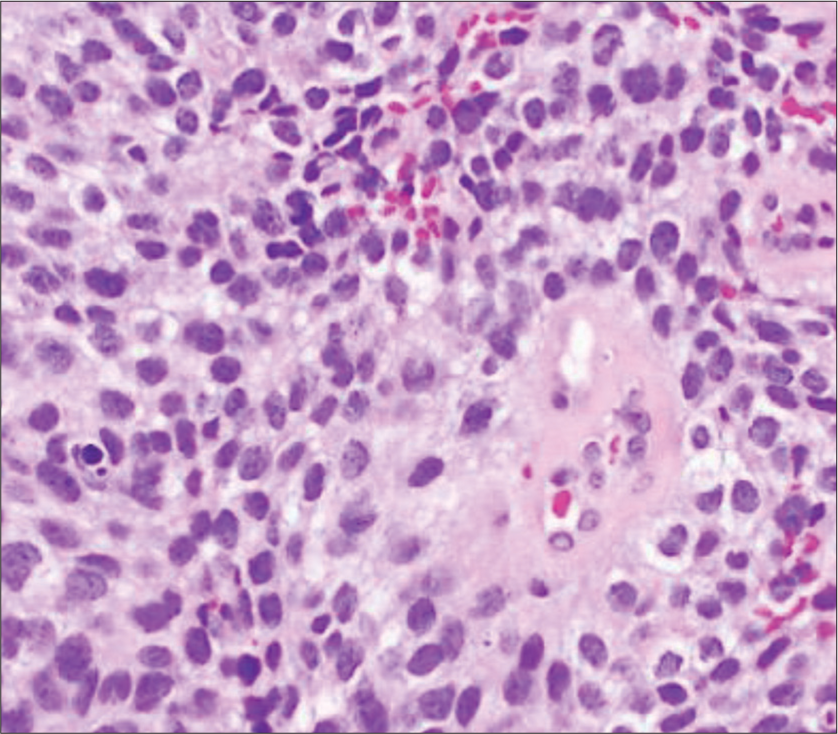
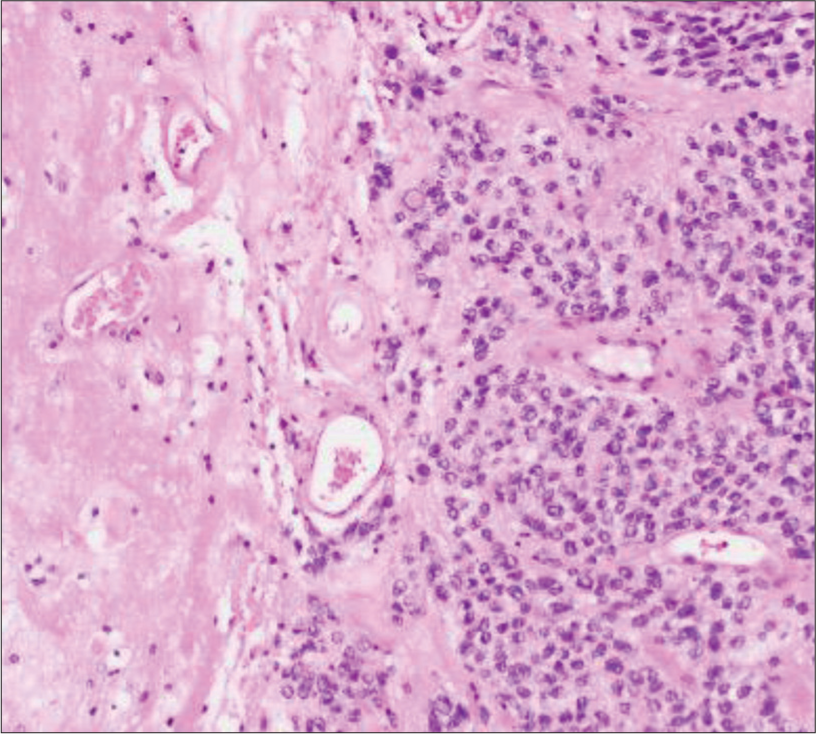
Microscopic analysis of the second recurrence [
Figure 5:
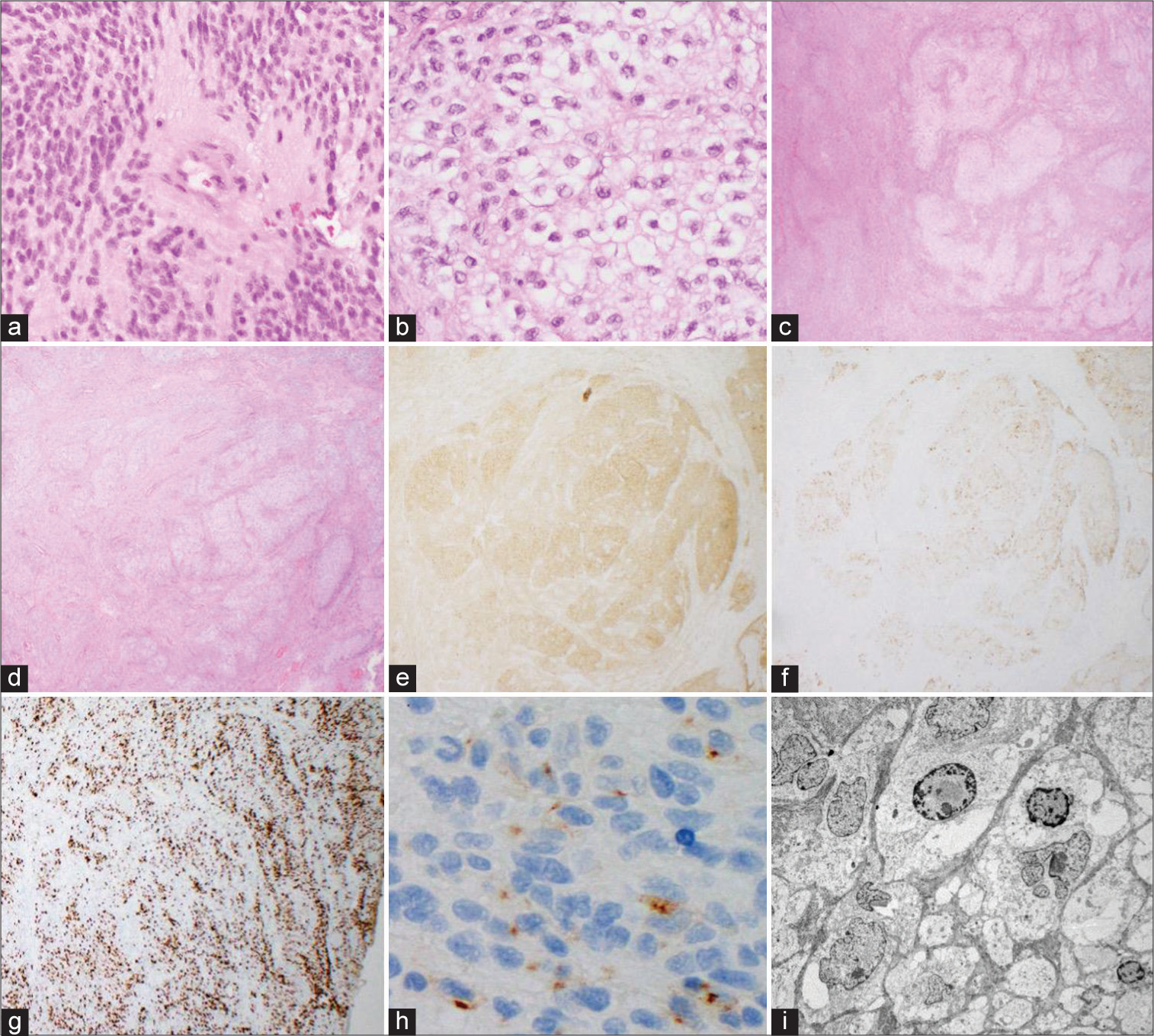
Third resection. High-power views of tumor with typical ependymoma features including relatively uniform ovoid cells with perivascular pseudorosettes (a; H&E, 200x) and areas with prominent clear cell change (b; H&E, 200x). Low power views (c-g, 20x) showing large nodules of tumor with clear cell morphology on H&E (c-d). A large nodule with clear cell morphology seen on H&E (d) is strongly positive for synaptophysin (e) and weakly positive for GFAP (f). Ki67 proliferation index is markedly elevated (g). EMA shows characteristic dot-like staining pattern (h; 400x). Transmission electron microscopy of clear cell regions with few poorly-formed intercellular junctions and intracellular glial-type filaments (i; magnification: 2500x).
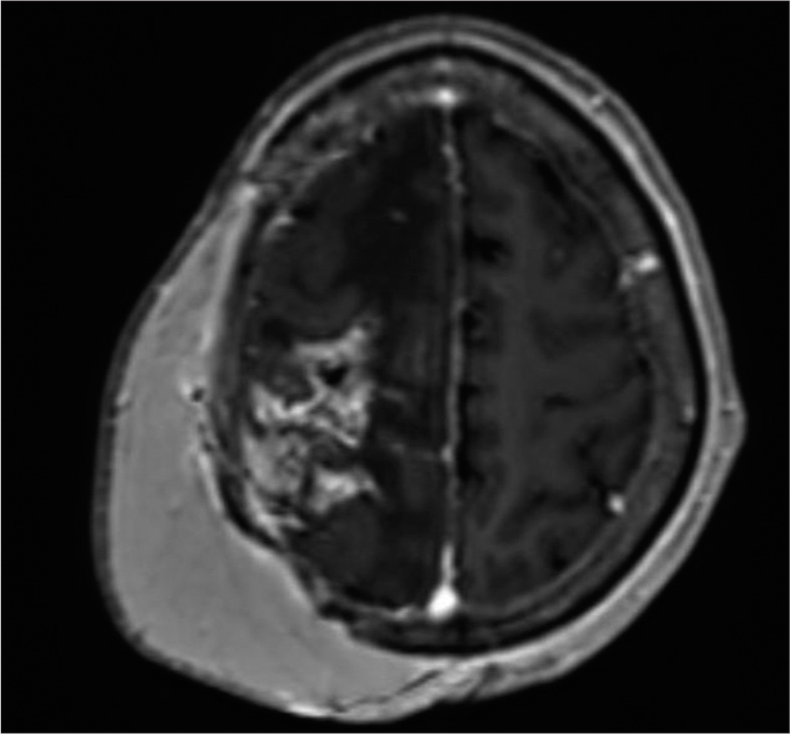
These findings were presented at tumor board and radiation therapy was recommended. Therapy was delayed in part due to her limited means of transportation. Planning MRI 3 months postoperatively showed an area of intense enhancement within the right frontoparietal vertex, concerning for a third recurrence. She received 3000 cGy in 10 fractions to the visualized tumor using image-guided intensity modulated radiation therapy (IG-IMRT). Two months later, there were moderately increased size and degree of enhancement of the affected area, as well as development of multiple satellite nodules. After another 2 months, the mass had demonstrated rapid growth with significant mass effect as well as necrotic bubbly enhancement of the perirolandic and deep white matter consistent with rapid tumor progression and radiation necrosis, respectively [
Her case was discussed at tumor board. Although she could lift her left arm, she had no functional use of the hand or lower extremity. A fourth resection was recommended to maximize her survival and allow for potential enrollment in a clinical trial. The resection was performed with neuronavigation, SSEPs, motor mapping, intraoperative microscope, ultrasonic aspiration, and plastic surgery management of her scalp flap. Intraoperatively, the tumor had a firm, gray and red center with an infiltrative border. After gross resection of the lesion, ultrasonic aspiration was used to remove all remaining lesion, with additional guidance based on neuronavigation. Pathology revealed an ependymal neoplasm and necrosis of brain parenchyma, compatible with radiation necrosis [
DISCUSSION
Before our understanding of the molecular underpinnings of ependymomas, prognosis was largely influenced by an amalgam of features that include histopathologic grading (e.g., anaplasia), as well as tumor size, location, and potential for gross total resection. Recent studies indicate that molecular subgrouping is superior to histopathological grading for risk stratification and predicting outcome.[
Although rare, several reports of radiation-induced ependymomas exist in the literature.[
Patients with ependymomas containing the C11orf95-RELA fusion are thought to have a worse outcome,[
This tumor demonstrated extensive immunoreactivity for synaptophysin, as well as NeuN and INSM1, suggesting the presence of neural differentiation, though this was not confirmed on electron microscopy. Neural differentiation in ependymoma is rare and, when it does occur, it is usually a minor component comprising focal patchy areas or small neuropil islands interspersed among primarily glial components usually within a supratentorial neoplasm.[
CONCLUSION
Whereas most documented reports of neural differentiation in ependymal neoplasms typically feature small islands of synaptophysin-positive neuropil, this case reports extensive synaptophysin immunoreactivity in an ependymoma with a ZFTA-RELA fusion that had undergone malignant transformation from a lower grade ependymoma with clear cell features diagnosed 16 years earlier. The presence of extensive synaptophysin positivity in ependymoma does not appear to confer a favorable outcome.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Andreiuolo F, Puget S, Peyre M, Dantas-Barbosa C, Boddaert N, Philippe C. Neuronal differentiation distinguishes supratentorial and infratentorial childhood ependymomas. Neuro Oncol. 2010. 12: 1126-34
2. De Bont JM, Packer RJ, Michiels EM, Den Boer ML, Pieters R. Biological background of pediatric medulloblastoma and ependymoma: A review from a translational research perspective. Neuro Oncol. 2008. 10: 1040-60
3. Ellison DW, Kocak M, Figarella-Branger D, Felice G, Catherine G, Pietsch T. Histopathological grading of pediatric ependymoma: Reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011. 10: 7
4. Figarella-Branger D, Lechapt-Zalcman E, Tabouret E, Jünger S, De Paula AM, Bouvier C. Supratentorial clear cell ependymomas with branching capillaries demonstrate characteristic clinicopathological features and pathological activation of nuclear factor-kappaB signaling. Neuro Oncol. 2016. 18: 919-27
5. Gessi M, Giagnacovo M, Modena P, Elefante G, Gianno F, Buttarelli FR. Role of immunohistochemistry in the identification of supratentorial C11ORF95-RELA fused ependymoma in routine neuropathology. Am J Surg Pathol. 2019. 43: 56-63
6. Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013. 12: 86
7. Jünger ST, Andreiuolo F, Mynarek M, Wohlers I, Rahmann S, Klein-Hitpass L. CDKN2A deletion in supratentorial ependymoma with RELA alteration indicates a dismal prognosis: A retrospective analysis of the HIT ependymoma trial cohort. Acta Neuropathol. 2020. 140: 405-7
8. Khoo HM, Kishima H, Kinoshita M, Goto Y, Kagawa N, Hashimoto N. Radiation-induced anaplastic ependymoma with a remarkable clinical response to temozolomide: A case report. Br J Neurosurg. 2013. 27: 259-61
9. Kurimoto M, Nagai S, Hamada H, Tsuboi Y, Hayashi N, Kubota T. Malignant transformation of supratentorial clear cell ependymoma. Neuropathology. 2009. 29: 299-302
10. Leung C, Lingbeek M, Shakhova O, Liu J, Tanger E, Saremaslani P. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature. 2004. 428: 337-41
11. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
12. Malgulwar PB, Nambirajan A, Pathak P, Faruq M, Rajeshwari M, Singh M. C11orf95-RELA fusions and upregulated NF-KB signalling characterise a subset of aggressive supratentorial ependymomas that express L1CAM and nestin. J Neurooncol. 2018. 138: 29-39
13. Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013. 15: ii1-56
14. Ozawa T, Arora S, Szulzewsky F, Juric-Sekhar G, Miyajima Y, Bolouri H. A de novo mouse model of C11orf95-RELA fusion-driven ependymoma identifies driver functions in addition to NF-κB. Cell Rep. 2018. 23: 3787-97
15. Pajtler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015. 27: 728-43
16. Parker M, Mohankumar KM, Punchihewa C, Weinlich R, Dalton JD, Li Y. C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature. 2014. 506: 451-5
17. Pietsch T, Wohlers I, Goschzik T, Dreschmann V, Denkhaus D, Dörner E. Supratentorial ependymomas of childhood carry C11orf95-RELA fusions leading to pathological activation of the NF-κB signaling pathway. Acta Neuropathol. 2014. 127: 609-11
18. Rodriguez FJ, Scheithauer BW, Robbins PD, Burger PC, Hessler RB, Perry A. Ependymomas with neuronal differentiation: A morphologic and immunohistochemical spectrum. Acta Neuropathol. 2007. 113: 313-24
19. Spallone A, Marchione P, Di Capua M, Belvisi D. Radiation-induced anaplastic ependymoma mimicking a skull base meningioma: A case report. Exp Ther Med. 2016. 11: 455-7
20. Tauziède-Espariat A, Siegfried A, Nicaise Y, Kergrohen T, Sievers P, Vasiljevic A. Supratentorial non-RELA, ZFTA-fused ependymomas: A comprehensive phenotype genotype correlation highlighting the number of zinc fingers in ZFTA-NCOA1/2 fusions. Acta Neuropathol Commun. 2021. 9: 135
21. 22. Zhu JJ, Jillette N, Li XN, Cheng AW, Lau CC. C11orf95-RELA reprograms 3D epigenome in supratentorial ependymoma. Acta Neuropathol. 2020. 140: 951-60