- Department of Neurosurgery, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India
- Department of Pathology, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India
Correspondence Address:
Sarbjit S. Chhiber
Department of Neurosurgery, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India
DOI:10.4103/sni.sni_374_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Uday S. Raswan, Irfan Bhat, Nuzhat Samoon, Sajad H. Arif, Masood Laharwal, Sarbjit S. Chhiber, Altaf Umar Ramzan. Supratentorial extraparenchymal schwannoma mimicking parasagittal meningioma: A rare case report. 26-Sep-2017;8:228
How to cite this URL: Uday S. Raswan, Irfan Bhat, Nuzhat Samoon, Sajad H. Arif, Masood Laharwal, Sarbjit S. Chhiber, Altaf Umar Ramzan. Supratentorial extraparenchymal schwannoma mimicking parasagittal meningioma: A rare case report. 26-Sep-2017;8:228. Available from: http://surgicalneurologyint.com/surgicalint-articles/supratentorial-extraparenchymal-schwannoma-mimicking-parasagittal-meningioma-a-rare-case-report/
Abstract
Background:Intracranial schwannomas not related to cranial nerves are very rare. Young age, no known history of neurofibromatosis, and seizure as initial symptom have been reported to be associated with intraparenchymal schwannoma.
Case Description:We report a case of supratentorial parasagittal schwannoma in the right frontal region presenting with seizure episode in a 70-year-old man. Computed tomography and magnetic resonance imaging showed a right frontal solid, enhancing extra-axial lesion based on anterior and middle third junction of superior sagittal sinus. The preoperative diagnosis was right parasagittal meningioma, however, the microscopic examination of the mass showed the characteristic pattern of cellular Antony A pattern. Immunohistocemically, the tumor stained positive for S-100 protein but negatively for epithelial membrane antigen and glial fibrillary acidic protein. These findings are consistent with schwannoma. Cysts, calcification, and peritumoral edema are common in intracerebral schwannoma, which were not seen in our case.
Conclusion:On the basis of clinical presentation and radiological appearances, schwannoma in unusual sites can easily be mistaken for meningiomas; immunochemistry plays an important role in differentiating them. Till date, to the best of our knowledge, this is the second reported case of schwannoma mimicking meningioma in parasagittal location.
Keywords: Immunohistochemistry, parasagittal meningioma, supratentorial schwannoma
INTRODUCTION
Schwannomas represent approximately 8% of all intracranial tumors predominantly arising from the vestibular portion of the 8th cranial nerve and, less commonly with descending order of frequency, from 5th, 9th, 10th, and 7th cranial nerves.[
CASE REPORT
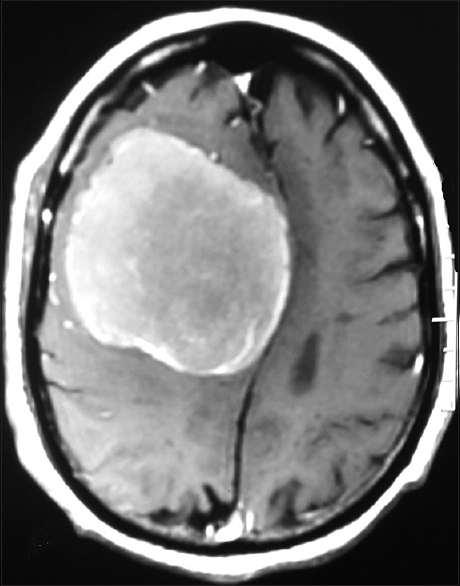
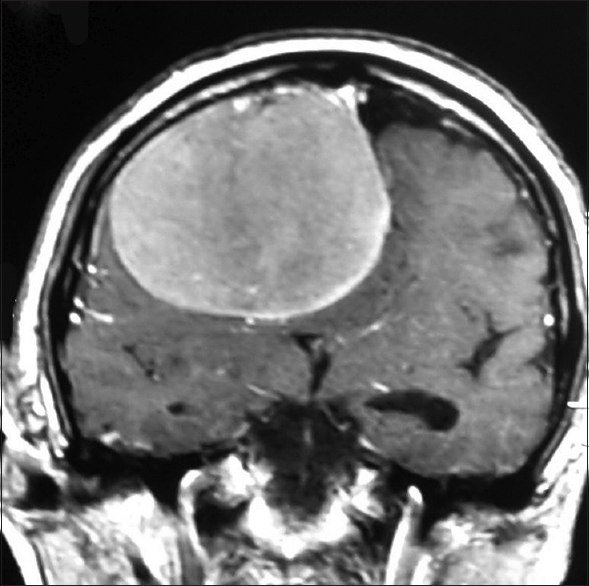
A 70-year-old man presented after a generalized tonic–clonic seizure. Residual left leg weakness was noted after the seizure. Neurological examination confirmed left lower limb weakness with grade 3/5 power. He had no other neurological deficits. Magnetic resonance imaging (MRI) revealed a large well-defined dural-based extra-axial lesion that was hypointense on T1 weighted and iso-hyperintense on T2-weighted images along the right frontal convexity causing mass effect and contralateral midline shift. Post-gadolinium images showed homogenous enhancement of the lesion based on the lateral superior sagittal sinus wall at the junction of anterior and middle third and on adjacent falx [Figures
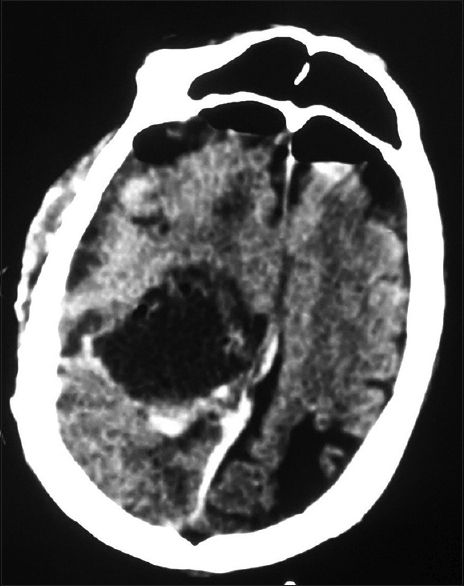
During surgery, a well-defined mildly vascular, firm, grayish, extra-axial tumor measuring 8 cm × 5 cm was found. In contrast to meningioma, note was made of more soft and suckable tumor, more dirty grayish color, and plenty of thin fibrous septae radiating towards the flimsy capsule dividing the tumor into infinite number of small compartments which could be easily emptied with suction. It was attached to the right inferolateral wall of the sinus and was growing between the two leaves of superior-lateral angle of sinus as a very thin sheet. There was no attachment of tumor capsule to the convexity dura. Gross total resection of the tumor was achieved (Simpson grade 2) which was confirmed by immediate postoperative computed tomography (CT) scan [
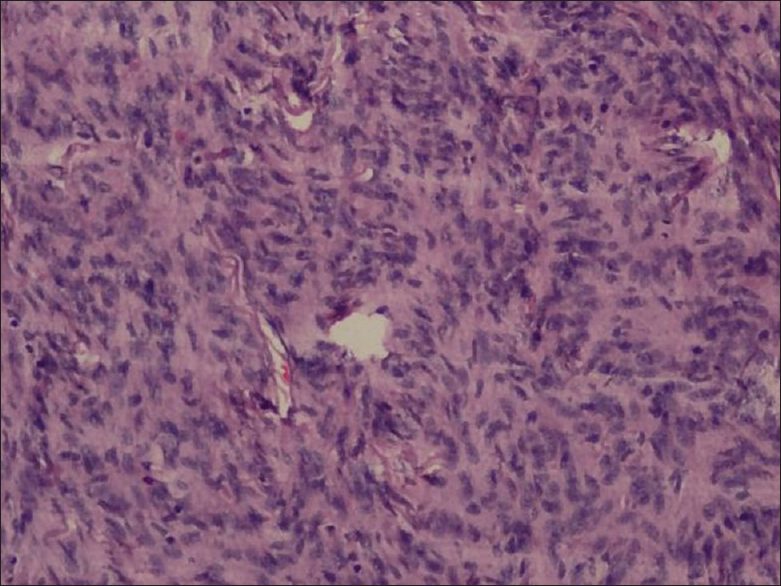
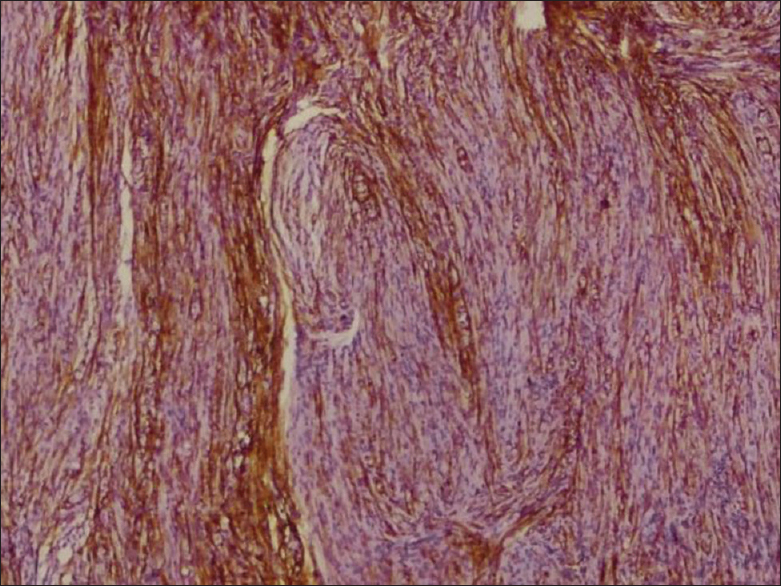
Microscopically, on hematoxylin and eosin staining, spindle cells arranged in fascicles forming verrocay bodies at places were seen [
The power of the left lower limb improved to normal after the operation. At follow-up 1 year later the patient remained well with no evidence of recurrence.
DISCUSSION
Intracranial schwannomas not related to cranial nerves are very rare and represent less than 1%. As reported elsewhere, approximately 70 cases have been reported so far.[
The location and size of the intracranial schwannoma decide the varied clinical manifestations. Most present with headache, seizures, and focal deficits. Our patient presented with a history of seizures. The typical MRI characteristics of meningioma consist of isointensity to slight hypointensity on T1-weighted sequence and isointensity to slight hyperintensity on T2 sequence. After contrast administration, meningiomas typically demonstrate avid, homogenous enhancement.[
Microscopically, tissue analysis reveals areas of nuclear palisading, characteristic of schwannoma and dense, cellular tumor alternating with loosely textured myxoid tumor is present in equal portions, consistent with Antoni type A and Antoni type B tissue.[
The histogenesis of intracranial schwannomas not arising from cranial nerves is still unclear as schwann cells are not normally present in the cerebral parenchyma. Many theories have been proposed to explain the possible mechanism underlying the histogenesis and origin of these rare tumors. There are two common theories. One suggests a developmental origin according to which aberrant schwann cells in the brain parenchyma may occur due to the transformation of the mesenchymal pial cells or from displaced neural crest cells that form the foci of schwann cells.[
Young age, no known history of neurofibromatosis, and seizure as initial symptom have been reportedly found associated with intraparenchymal schwannoma.[
The treatment protocol of intracerebral schwannomas is total excision; however, it depends on the location of tumors. Complete relief of clinical symptoms and signs is mostly achieved after total or radical surgical removal.[
In summary, on the basis of clinical presentation and radiological appearances, schwannoma in unusual sites can easily be mistaken for meningiomas. Light microscopy may also reveal similar findings. Immunohistochemical techniques with a battery of antibodies offer a greater diagnostic specificity. Our case demonstrates only the second rare case of a supratentorial schwannoma mimicking a meningioma in parasagittal location.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Gibson AAM, Hendrick EB, Connen PE. Intracerebral schwannoma: Report of a case. J Neurosurg. 1996. 24: 552-7
2. Guha D, Kiehl TR, Krings T, Valiante TA. Intracerebral schwannoma presenting as classic temporal lobe epilepsy. J Neurosurg. 2012. 117: 136-40
3. Lee S, Park SH, Chung CK. Supratentorial intracerebral schwannoma: Its fate and proper management. J Korean Neurosurg Soc. 2013. 54: 340-3
4. Louw D, Sutherland G, Halliday W, Kaufmann J. Meningiomas mimicking cerebral schwannoma. J Neurosurg. 1990. 73: 715-9
5. Luan SH, Gao X, Sun LL, Huang FP. Frontal intraparenchymal schwannoma. Neurosciences. 2010. 15: 37-9
6. Ma L, Yang SX, Wang YR. Intracerebral schwannoma mimicking parasagittal meningioma. J Craniofac Surg. 2013. 24: 541-3
7. Menku A, Oktem IS, Kontas O, Akdemir H. Atypical intracerebral schwannoma mimicking glial tumor: Case report. Turk Neurosurg. 2009. 19: 82-5
8. Nelson E, Rennels M. Innervation of intracranial arteries. Brain. 1970. 93: 475-90
9. Perentes E, Rubinstein LJ. Immunohistochemical recognition of human nerve sheath tumours by anti-Leu 7 monoclonal antibody. Acta Neuropathol. 1985. 68: 319-24
10. Russels DS, Rubinstein LJ.editorsPathology of tumors of the nervous system. London: Edward Arnold; 1989. p. 776-7
11. Schnitt SJ, Vogel H. Diagnostic value of immunoperoxidase staining for epithelial membrane antigen. Am J Surg Pathol. 1986. 10: 640-9
12. Sharma MC, Karak AK, Gaikwad SB, Mahapatra AK, Mehta VS, Sudha K. Intracranial intraparenchymal schwannomas: A series of eight cases. J Neurol Neurosurg Psychiatry. 1996. 60: 200-3
13. Sobel RA, Michaud J. Microcystic meningioma of the falx cerebri with numerous palisaded structures: An unusual pattern mimicking schwannoma. Acta Neuropathol. 1985. 68: 256-8
14. Takei H, Schmiege L, Buckleair L, Goodman JC, Powell SZ. Intracerebral schwannoma clinically and radiologically mimicking meningioma. Pathol Int. 2005. 55: 514-9
15. Watts J, Box G, Galvin A, Brotchie P, Trost N, Sutherland T. Magnetic resonance imaging of meningiomas: A pictorial review. Insights Imaging. 2014. 5: 113-22
16. Winek RR, Schethauer BW, Wick MR. Meningioma, meningeal hemangiopericytoma (angioblastic meningioma), peripheral hemangiopericytoma and acoustic schwannoma: A comparative immunohistochemical study. Am J Surg Pathol. 1989. 13: 251-61
17. Zagardo MT, Castellani RJ, Rees JH, Rothman MI, Zoarski GH. Radiologic and pathologic findings of intracerebral schwannoma. AJNR Am J Neuroradiol. 1998. 19: 1290-3