- Department of Neurosurgery, Fukuoka Children’s Hospital, Fukuoka, Japan
- Department of Neurosurgery, Harasanshin Hospital, Karatsu, Saga,
- Department of Psychiatry, Shourai Hospital, Karatsu, Saga,
- Department of Neurosurgery, Graduate School of Medical Sciences, Fukuoka, Japan.
- Department of Neurosurgery, Kyushu University, Fukuoka, Fukuoka, Japan.
Correspondence Address:
Nobuya Murakami, Department of Neurosurgery, Fukuoka Children’s Hospital, Fukuoka, Japan.
DOI:10.25259/SNI_340_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takato Morioka1,2, Nobuya Murakami1, Satoshi O. Suzuki3, Nobutaka Mukae4, Takafumi Shimogawa5, Ai Kurogi1, Tadahisa Shono2, Masahiro Mizoguchi4. Surgical histopathology of a filar anomaly as an additional tethering element associated with closed spinal dysraphism of primary neurulation failure. 27-Jul-2021;12:373
How to cite this URL: Takato Morioka1,2, Nobuya Murakami1, Satoshi O. Suzuki3, Nobutaka Mukae4, Takafumi Shimogawa5, Ai Kurogi1, Tadahisa Shono2, Masahiro Mizoguchi4. Surgical histopathology of a filar anomaly as an additional tethering element associated with closed spinal dysraphism of primary neurulation failure. 27-Jul-2021;12:373. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11000
Abstract
Background: Closed spinal dysraphism of primary neurulation failure could be associated with filar anomalies, such as filar lipoma or thickened and tight filum terminale (TFT), resulting from impaired secondary neurulation. Retained medullary cord (RMC) is a remnant of the cavitary medullary cord originating from the secondary neurulation failure. Some filar lipomas are known to contain a central canal-like ependyma-lined lumen with surrounding neuroglial tissues (E-LC w/NGT), that is, a characteristic histopathology of RMC. To clarify the embryological background of these filar anomalies, we evaluated the histopathological findings.
Methods: Among 41 patients with lesions of primary neurulation failure who underwent initial untethering surgery, the filum including cord-like structure (C-LS) was additionally resected in 10 patients (five dorsal and transitional lipomas; five limited dorsal myeloschisis). We retrospectively analyzed the clinical, neuroradiological, intraoperative, and histopathological findings.
Results: Among 10 patients, two patients were diagnosed with RMC based on morphological features and intraoperative neurophysiological monitoring. The diagnosis of filar lipoma was made in six patients, since various amounts of fibroadipose tissue were histopathologically noted in the filum. Two patients were diagnosed with TFT, since the filum was composed solely of fibrocollagenous tissue. E-LC w/NGT was noted not only in both C-LSs of RMCs but also in two out of six fila both with filar lipomas and fila with TFTs.
Conclusion: These findings provide further evidence for the idea that entities, such as filar lipoma, TFT, and RMC, can be considered consequences of a continuum of regression failure occurring during late secondary neurulation.
Keywords: Central canal, Ependyma, Filar type lipoma, Neuroglial core, Primary neurulation, Retained medullary cord, Secondary neurulation
INTRODUCTION
The central nervous system and vertebrae are formed during the neurulation process that occurs early in embryonic life and are responsible for the transformation of the flat neural plate into the neural tube. Primary neurulation allows the formation of the brain and the spinal cord down to the junction between the S1 and S2 segments, whereas secondary neurulation is responsible for the formation of the spinal cord segments distal to the S1-2 junction. Although primary neurulation is relatively well understood, less is known about the process of secondary neurulation or its clinical importance.[
Failure of primary neurulation leads to open neural tube defects, including myelomeningocele and myeloschisis, or closed neural tube defects, including spinal lipoma of dorsal and transitional type, limited dorsal myeloschisis (LDM), and congenital dermal sinus (CDS). When the secondary neurulation process is impaired, the result is either a defect of formation that leads to an absent conus and a short spinal cord, a condition known as caudal agenesis,[
RMC is a newly defined entity of closed spinal dysraphism that is thought to originate from an almost complete arrest of apoptosis during the last phase of secondary neurulation.[
When there are alterations present in both the primary and secondary neurulation, we can find the coexistence of dysraphism that presents with elements from the two forms of neural tube defects.[
MATERIALS AND METHODS
From January 2015 to December 2020, 41 patients with closed spinal dysraphic lesions of primary neurulation failure underwent initial untethering surgery at Fukuoka Children’s Hospital and related hospitals under supervision of the first author (T.M.). There were 16 spinal lipomas at the lumbosacral region (six dorsal lipomas and 10 transitional lipomas), 23 LDMs, and two CDSs with dermoid/epidermoid cysts. Split cord malformation, which is caused by developmental failure of the notochord, was not involved in the present study. At our institute, the classification of spinal lipoma was based on Arai et al.[
In all patients, preoperative and postoperative magnetic resonance imaging (MRI), including three-dimensional T1-weighted spoiled gradient-recalled echo images or variable flip angle three-dimensional turbo spin echo T1-weighted images, and three-dimensional heavily T2-weighted images, were performed as described in our previous report.[
The decision to sever the filum was based on whether the filum was involved as an additional tethering element, judging from the preoperative MRI and intraoperative findings. Based on the intraoperative morphological findings, the fila were classified into three types; CL-S, fatty filum (short fatty filum, tight fatty filum, or fatty filum), and short or tight filum. Before cutting the filum, for IONM, we confirmed that no compound muscle action potentials (CMAPs) were evoked at the external anal sphincter, hamstrings, and gastrocunemius muscles following the stimulation of the filum to be severed. In cases of suspected RMC, the border between the true cord and nonfunctional C-LS was determined by tracing the evoked CMAPs by stimulation in the rostrocaudal direction, beginning from the functional cord, and proceeding to the nonfunctional C-LS, as described previously.[
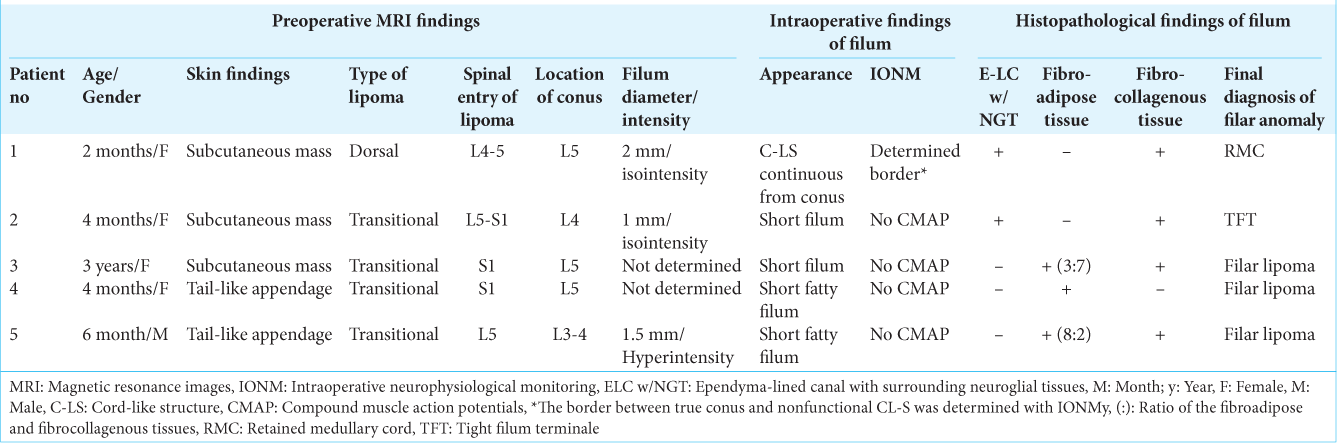
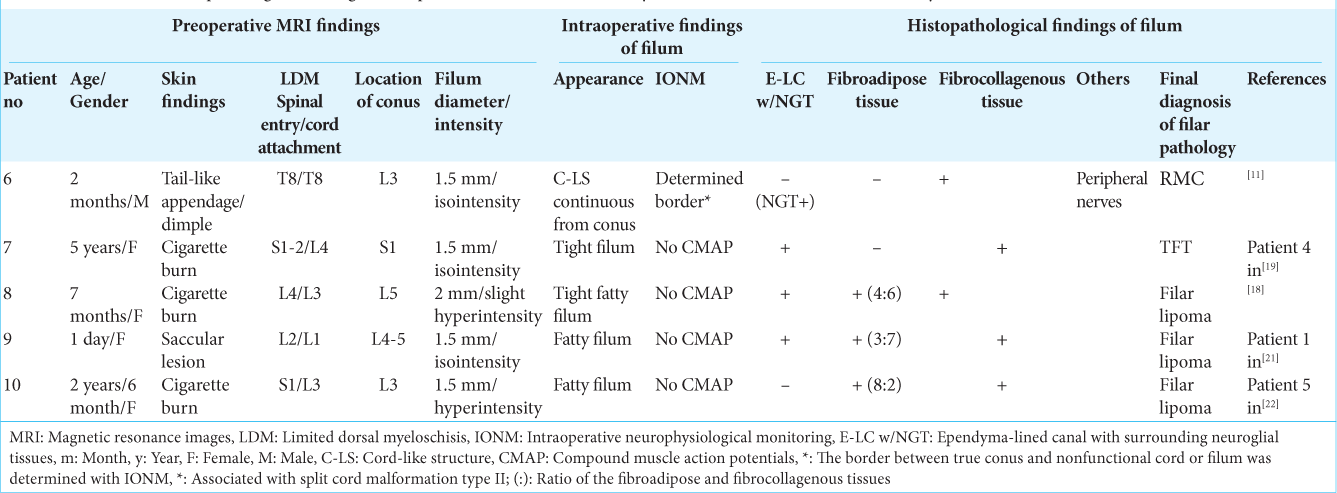
Among these 41 patients, the filum was additionally resected in 10 patients. Of the six patients with dorsal lipomas, the filum was resected in Patient 1 [
In all patients, the filum was resected as a column and placed in formalin. Routinely prepared histopathological sections were stained with hematoxylin and eosin or immunostained for glial fibrillary acidic protein (GFAP) and S-100 protein as part of the standard diagnostic analysis. Histopathological examination was performed with particular attention to the presence or absence of fibroadipose tissue, fibrocollagenous tissue, and E-LC w/NGT. When fibroadipose and fibrocollagenous tissues were present, the ratio of both was visually determined. The final differential diagnosis of filar lipoma from TFT was made with the histopathological presence of fibroadipose tissue. In all 10 patients, no postoperative neurological worsening was noted. We retrospectively analyzed the clinical, neuroradiological, intraoperative, and histopathological findings of these 10 patients.
RESULTS
On histopathological examination of the resected filum including C-LS, E-LC w/NGT was noted in two out of five patients (Patients 1 and 2) with dorsal [
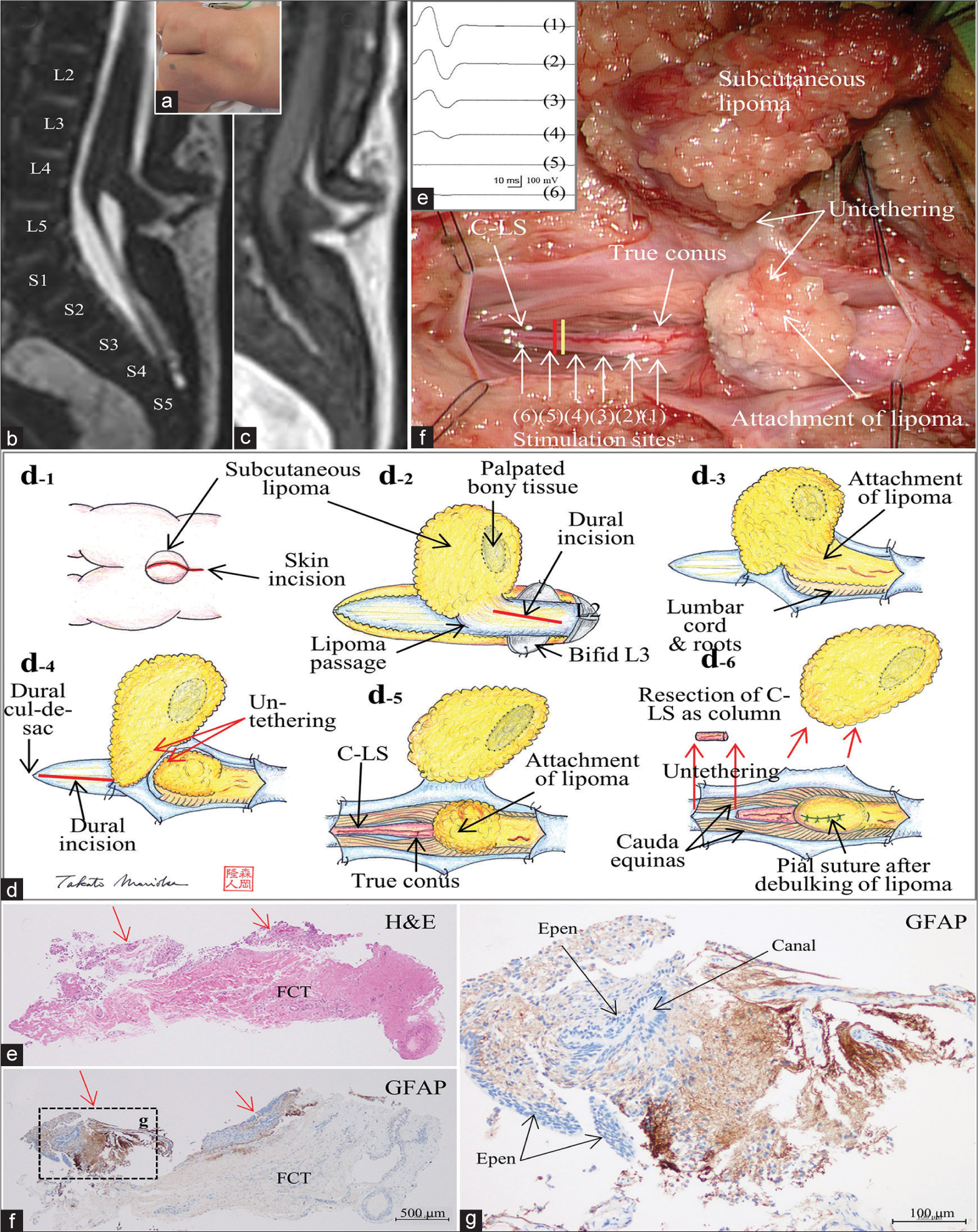
Figure 1:
(Patient 1) (a) Photograph showing a subcutaneous lipoma at the lumbosacral region. Sagittal views of a three-dimensional heavily T2-weighted image (3D-hT2WI, slice thickness of 1.25 mm) (b) and a 3D variable flip angle T1-weighted image (3D-T1WI, slice thickness of 1.25 mm) (c) depict a low-lying conus medullaris at the vertebral level of L5 that is tethered by dorsal lipoma, and cord-like structure (C-LS) extends from the conus and terminates at the dural cul-de-sac at S4-5 level without much tapering. Schematic drawings (d) and microscopic view of the operative findings (e), and intraoperative neurophysiological monitoring (f). (d-1) A linear skin incision on the subcutaneous lipoma and a subsequent incision on the rostral side are made. (d-2) Passage of the lipoma through the dura mater is noted at L4 level, and the dura incision is made on the rostral site to the lipoma passage. (d-3) With the dura opened, the intradural component of lipoma is exposed. (d-4) Lumbar cord is untethered from the lipoma. The dura incision is made on the caudal site to the lipoma passage and extended to the dural cul-de-sac. (d-5, e) With the dura opened, C-LS is continuous from the conus to the dural cul-de-sac. The exact border between the C-LS and the true conus is determined by tracing the evoked compound muscle action potentials of the external anal sphincter muscle (f) with direct stimulation starting from the functional portion of the conus (1-4) and continuing to the nonfunctional portion of the C-LS (5 and 6), and indicated by the yellow line in (e). C-LS is severed immediately caudal to the exact border (indicated with red line in (e)). (d-6) Caudal end of the C-LS is also severed, and the C-LS is resected as column. Finally, the lipoma is debulked, and the pial surface is reconstructed with sutures. (e-g) Photomicrograph of longitudinal sections of the C-LS stained with hematoxylin and eosin (H and E) (e) and immunostained for glial fibrillary acidic protein (GFAP) (f and g). A higher magnification view of the area indicated by the dotted square in (f) is shown in (g). In the marginal part of the fibrocollagenous tissue of the C-LS, a central canal-like, ependyma (Epen)-lined canal with surrounding GFAP-immunopositive neuroglial tissues is noted (indicated with red arrows in (e) and (f)). No adipose tissue is noted.
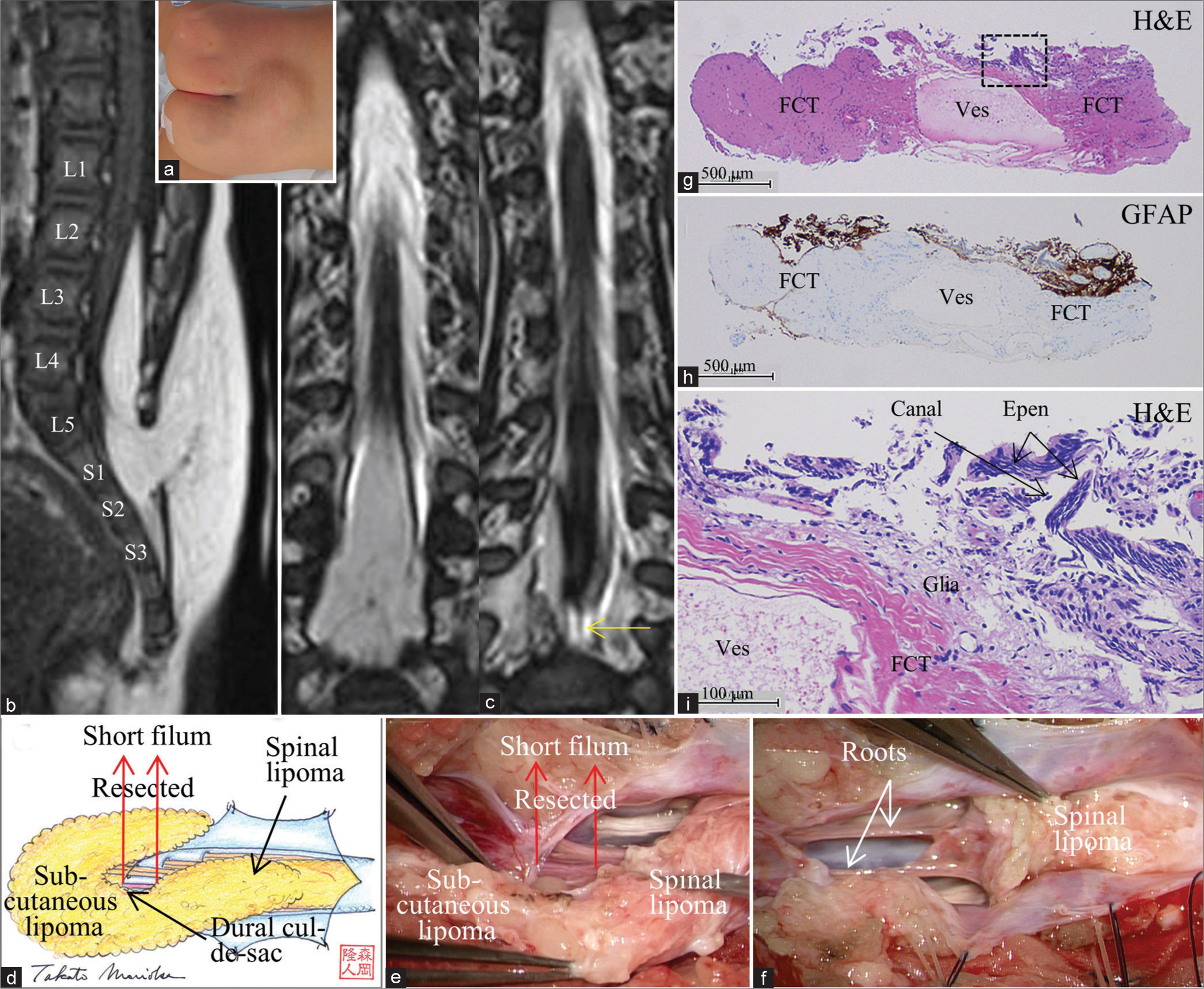
Figure 2:
(Patient 2) (a) Photograph showing a subcutaneous lipoma at the lumbosacral region. Sagittal view of 3D-T1WI (slice thickness of 1.25 mm) (b) and serial coronal views of 3D-hT2WI (slice thickness of 1.25 mm) (c) depict a low-lying conus at the vertebral level of L3-4 that is tethered by the spinal lipoma of transitional type and short filum, which is barely seen (b-2, yellow arrow). Schematic drawings (d) and microscopic view of the operative findings (e and f). (d and e) With the dura opened, the subcutaneous lipoma is continuous with the spinal lipoma. Following untethering of the cord from the lipoma, the short filum (red arrows) is exposed, severed immediately rostral and caudal to the dural cul-de-sac and the spinal lipoma, respectively, and resected as column. (f) Following resection of the filum and debulking of the lipoma, the conus is slightly elevated, and spinal roots, which was involved with transitional lipoma, are stretched. (g-i) Photomicrograph of longitudinal sections of the filum stained with H and E (g and i) and immunostained for glial fibrillary acidic protein (h) A higher magnification view of the area indicated by the dotted square in (g) is shown in (i). In the marginal part of the fibrocollagenous tissue containing large vessel (ves), E-LC w/NGT is noted. No adipose tissue is observed.
Figure 3:
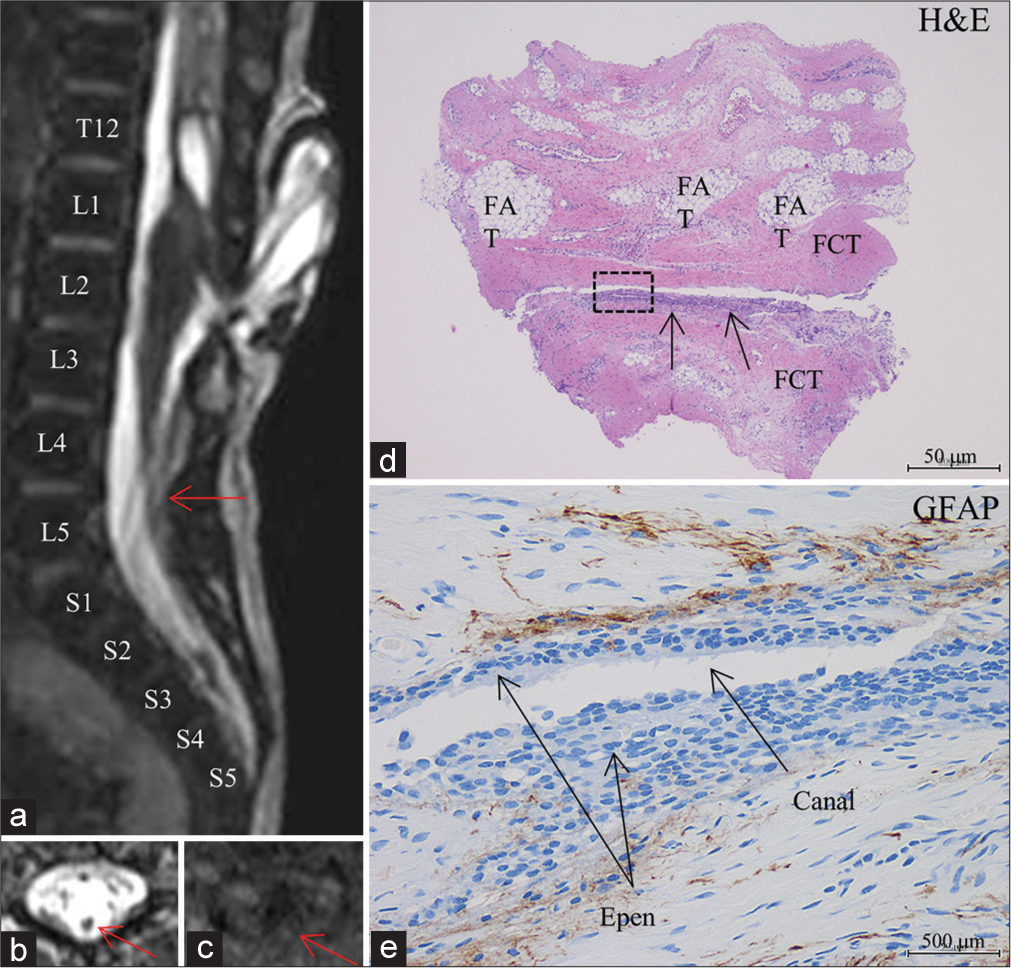
(Patient 9) (a) Sagittal view of 3D-hT2WI (slice thickness of 1.25 mm) depicts a low-lying conus at the vertebral level of L4-5 that is tethered by the stalk of limited dorsal myeloschisis (LDM), which starts at the dome of the meningocele sac, travels down in the sac, enters the spinal canal, and join the dorsal cord, and terminal filum of 1.5 mm in diameter. A syringomyelic cavity is noted immediately rostral to the cord LDM stalk attachment. This image is 1.25 mm lateral to the left side to the image in Figure 1b of our previous report.[
Among these eight patients with positive E-LC w/NGT, the final diagnosis of RMC was made in two patients (Patient 1 with dorsal lipoma and Patient 6 with LDM), based on the morphological features and IONM findings [
The final diagnosis of filar lipoma was made in eight patients (Patients 3-5 with transitional lipoma and Patients 8-10 with LDM), since various amounts of fibroadipose tissue were histopathologically noted in the filum. There was only one filum in Patient 4 composed solely of fibroadipose tissue; however, the remaining seven patients had a mixture of fibroadipose and fibrocollagenous tissues. When the ratio of fibroadipose and fibrocollagenous tissues was 4:6 or more, the filum was demonstrated as high intensity on T1-weighted MRI and looked like fatty filum in the intraoperative view. However, when the ratio was 3:7, the filum was demonstrated as isointensity on T1-weighted MRI, as seen in Patient 9 [
In the remaining two patients (Patient 2 with transitional lipoma and Patient 7 with LDM), the final diagnosis of TFT was made, since the filum was composed solely of dense fibrocollagenous tissue without fibroadipose tissue [
DISCUSSION
In this study, E-LC w/NGT was histopathologically observed in 6 (60%) out of 10 fila, including C-LSs, which were resected, as an additional tethering element, in 41 patients with closed spinal dysraphism of primary neurulation failure. However, the histopathological confirmation of E-LC w/NGT alone does not lead to the diagnosis of RMC, and the IONM confirmation of the functional conus and nonfunctional C-LS is essential.[
In the present study, E-LC w/NGT was also noted in two out of six filar lipomas and in both TFTs. While E-LC w/NGT was reported to be present in the surgically resected filum associated with tethered cord syndrome[
Preoperative differential diagnosis is even more difficult. For example, it is generally accepted that differential diagnosis between the lipoma and TFT can be made from the intensity on preoperative T1-weighted MRI;[
This study was a retrospective study with a small number of the patients. Although further studies with large numbers of patients are needed, this study revealed that E-LC w/ NGT was noted not only in the two C-LS of RMCs but also in four fila with filar lipomas and TFTs. These findings provide further evidence for the idea that entities such as filar lipoma, TFT, and RMC can be considered consequences of a continuum of regression failure occurring during late secondary neurulation.
CONCLUSION
Our findings provide further evidence for the idea that entities, such as filar lipoma, TFT, and RMC, can be considered consequences of a continuum of regression failure occurring during late secondary neurulation.
Ethics statement
The authors confirm that written informed consent was obtained from the families of the infants described in this report.
The authors declare that this work complies with the guidelines for human studies, and the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Research Foundation of Fukuoka Children’ Hospital.
Conflicts of interest
There are no conflicts of interest.
References
1. Arai H, Sato K, Okuda O, Miyajima M, Hishii H, Nakanishi H. Surgical experience of 120 patients with lumbosacral lipomas. Acta Neurochir (Wien). 2001. 143: 857-64
2. Choi BH, Kim RC, Suzuki M, Choe W. The ventriculus terminalis and filum terminate of the human spinal cord. Hum Pathol. 2019. 23: 916-20
3. Durdaĝ E, Börcek PB, Öcal Ö, Börcek AÖ, Emmez H, Baykaner MK. Pathological evaluation of the filum terminale tissue after surgical excision. Childs Nerv Syst. 2015. 31: 759-63
4. Gupta A, Rajshekhar V. Fatty filum terminale (FFT) as a secondary tethering element in children with closed spinal dysraphism. Childs Nerv Syst. 2018. 34: 925-32
5. Hashiguchi K, Morioka T, Fukui K, Miyagi Y, Mihara F, Yoshiura T. Usefulness of constructive interference in steady-state magnetic resonance imaging in the presurgical examination for lumbosacral lipoma. J Neurosurg. 2005. 103: 537-43
6. Hiraoka A, Morioka T, Murakami N, Suzuki SO, Mizoguchi M. Limited dorsal myeloschisis with no extradural stalk linking to a flat skin lesion: A case report. Childs Nerv Syst. 2018. 34: 2497-501
7. Kim KH, Lee JY, Wang KC. Secondary neurulation defects-1: Retained medullary cord. J Korean Neurosurg Soc. 2020. 63: 314-20
8. Kurogi A, Murakami N, Morioka T, Mukae N, Shimogawa T, Kudo K. Two cases of retained medullary cord running parallel to a terminal lipoma. Surg Neurol Int. 2021. 12: 112
9. Morioka T, Murakami N, Ichiyama M, Kusuda T, Suzuki SO. Congenital dermal sinus elements in each tethering stalk of coexisting thoracic limited dorsal myeloschisis and retained medullary cord. Pediatr Neurosurg. 2020. 55: 380-7
10. Morioka T, Murakami N, Kanata A, Tsukamoto E, Suzuki OS. Retained medullary cord associated with sacral subcutaneous meningocele and congenital dermal sinus. Childs Nerv Syst. 2020. 36: 423-7
11. Morioka T, Murakami N, Shimogawa T, Mukae N, Hashiguchi K, Suzuki SO. Neurosurgical management and pathology of lumbosacral lipomas with tethered cord. Neuropathology. 2017. 37: 385-92
12. Morioka T, Murakami N, Yanagida H, Yamaguchi T, Noguchi Y, Takahata Y. Terminal syringomyelia associated with lumbar limited dorsal myeloschisis. Childs Nerv Syst. 2020. 36: 819-26
13. Morioka T, Suzuki SO, Murakami N, Mukae N, Shimogawa T, Haruyama H. Surgical histopathology of limited dorsal myeloschisis with flat skin lesion. Childs Nerv Syst. 2019. 35: 119-28
14. Morioka T, Suzuki SO, Murakami N, Shimogawa T, Mukae N, Inoha S. Neurosurgical pathology of limited dorsal myeloschisis. Childs Nerv Syst. 2018. 34: 293-303
15. Morota N, Ihara S, Ogiwara H. New classification of spinal lipomas based on embryonic stage. J Neurosurg Pediatr. 2017. 19: 428-39
16. Mukae N, Morioka T, Suzuki SO, Murakami N, Shimogawa T, Kanata A. Two cases of large filar cyst associated with terminal lipoma: Relationship with retained medullary cord. World Neurosug. 2020. 142: 294-8
17. Murakami N, Morioka T, Hashiguchi K, Yoshiura T, Hiwatashi K, Suzuki SO. Usefulness of three-dimensional T1-weighted spoiled gradient-recalled echo and three-dimensional heavily T2-weighted images in preoperative evaluation of spinal dysraphism. Childs Nerv Syst. 2013. 29: 1905-14
18. Murakami N, Morioka T, Shimogawa T, Hashiguchi K, Mukae N, Uchihashi K. Retained medullary cord extending to a sacral subcutaneous menigocele. Childs Nerv Syst. 2018. 34: 527-33
19. Murakami N, Morioka T, Shimogawa T, Mukae N, Inoha S, Sasaguri T. Ependyma-lined canal with surrounding neuroglial tissues in lumboscaral lipomatous malformations: Relationship with retained medullary cord. Pediatr Neurosurg. 2018. 53: 387-94
20. Pang D, Chong S, Wang KC, di Rocco C, Pang D, Rutka JT.editors. Secondary neurulation defects-1: Thickened filum terminale, retained medullary cord. Textbook of Pediatric Neurosurgery. Switzerland: Springer; 2020. p.
21. Pang D, Zovickian J, Moes GS. Retained medullary cord in humans: Late arrest of secondary neurulation. Neurosurgery. 2011. 68: 1500-19
22. Pang D. Sacral agenesis and caudal spinal cord malformations. Neurosurgery. 1993. 32: 755-79
23. Sala F, Barone G, Tramontano V, Gallo P, Ghimenton C. Retained medullary cord confirmed by intraoperative neurophysiological mapping. Childs Nerv Syst. 2014. 30: 1287-91
24. Selçuki M, Vatansever S, Inan S, Erdemli E, Baĝdatoĝlu C, Polat A. Is a filum terminale with a normal appearance rarely normal?. Childs Nerv Syst. 2003. 19: 3-10
25. Shirozu N, Morioka T, Inoha S, Imamoto N, Sasaguri T. Enlargement of sacral subcutaneous meningocele associated with retained medullary cord. Childs Nerv Syst. 2018. 34: 1785-90