- Department of Orthopaedic Surgery, University of California Davis Medical Center, Sacramento, California, United States
- Department of Orthopedic Surgery, Boston Medical Center, One Boston Medical Center Place, Boston, Massachusetts, United States.
Correspondence Address:
Aziz Saade, Department of Orthopaedic Surgery, University of California Davis Medical Center, Sacramento, California, United States.
DOI:10.25259/SNI_41_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Aziz Saade1, Hayley M. Denwood2, Tony Tannoury2, Chadi Tannoury2. Surgical management of intramedullary cervical spinal sarcoidosis complicated by transient unilateral weakness: A case report. 08-Mar-2024;15:76
How to cite this URL: Aziz Saade1, Hayley M. Denwood2, Tony Tannoury2, Chadi Tannoury2. Surgical management of intramedullary cervical spinal sarcoidosis complicated by transient unilateral weakness: A case report. 08-Mar-2024;15:76. Available from: https://surgicalneurologyint.com/surgicalint-articles/12790/
Abstract
Background: Sarcoidosis, a multisystem inflammatory non-caseating granulomatous disease, can present with neurologic lesions in up to 10% of patients.
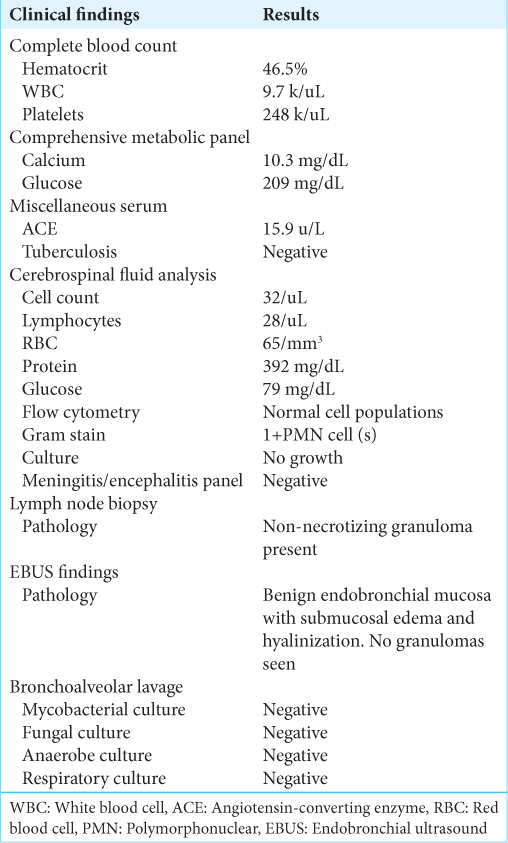
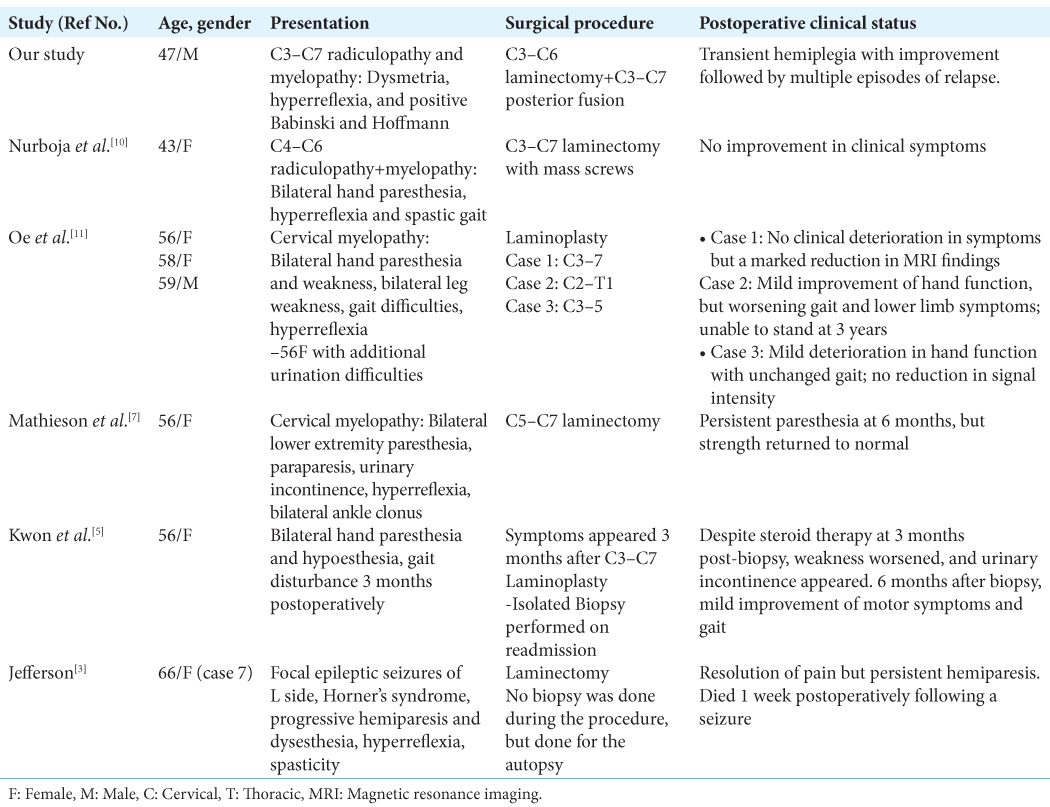
Case Description: A 57-year-old male presented with three months of worsening upper extremity radicular pain associated with dysmetria, hyperreflexia, bilateral Hoffman’s, and positive Babinski signs. The contrast magnetic resonance imaging (MRI) showed a diffuse T2 signal hyperintensity and T1-enhancing 2.5 cm lesion extending sagittally between C4 and C6. The cerebrospinal fluid analysis showed a high protein level and lymphocytic pleocytosis. A cardiac positron emission tomography scan was consistent with the diagnosis of cardiac sarcoidosis. With the diagnosis of multisystemic/probable neurosarcoidosis, the patient was unsuccessfully treated with intravenous methylprednisolone, followed by infliximab. Due to severe cord compression/myelopathy, a C3–C6 laminectomy and C3–C7 posterior spinal fusion were performed. Postoperatively, the patient developed a transient right-sided hemiparesis. Over nine postoperative months, the patient had four relapses of transient repeated episodes of paresis, although follow-up cervical MRI scans revealed adequate cord decompression with a stable intramedullary hyperintense lesion.
Conclusion: Patients with neurosarcoidosis respond unpredictably to surgical decompression and require prolonged medical care, which is often unsuccessful.
Keywords: Cervical intramedullary lesion, Laminectomy, Neurosarcoidosis
INTRODUCTION
Sarcoidosis, a chronic multisystem inflammatory non-caseating granulomatous disease of unknown etiology, can present with neurologic lesions in up to 10% of patients, including intramedullary or extramedullary spinal cord lesions.[
CASE PRESENTATION
Clinical findings
A 47-year-old African American male with a past medical history of glucose-6-phosphate dehydrogenase deficiency presented with three months of worsening bilateral upper extremity radicular pain/numbness and tingling radiating to both shoulders. On examination, he had bilateral hyperreflexia, bilateral Hoffmann’s and Babinski’s signs, all reflecting the presence of significant cervical myelopathy.
Radiological diagnosis of sarcoidosis
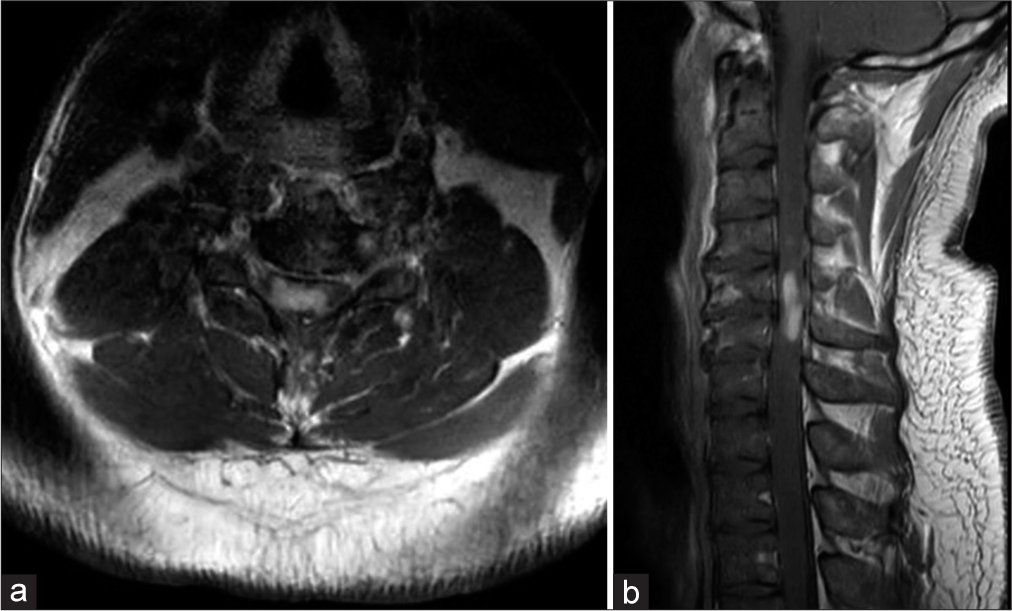
The cervical magnetic resonance imaging (MRI) demonstrated congenital spinal stenosis and a 2.5 cm intramedullary C4–C6 spinal cord lesion that enhanced with contrast on the T2 image and was diffusely hyperintense on the T2-weighted studies [
Initial failed medical management
With the initial diagnosis of multisystemic sarcoidosis/probable neurosarcoidosis, the patient was started on daily IV methylprednisolone (dose of 1 g). After developing a left punctate pontine stroke (i.e., resulting in left internuclear ophthalmoplegia), vertical nystagmus, and mild left ptosis, he was started on clopidogrel and aspirin. However, as the subsequent contrast brain MRI revealed pachymeningitis, leptomeningitis, and a T2-hyperintense lesion in the pons, the patient was subsequently started on infliximab (5 mg/kg) and referred to neurosurgery with the diagnosis of severe cervical myelopathy secondary to a cervical C4–C6 intramedullary neurosarcoid lesion.
Cervical surgery
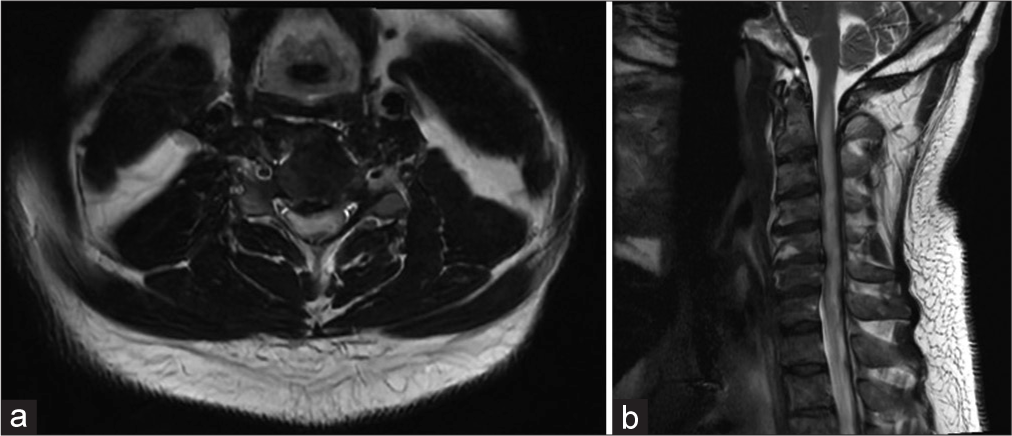
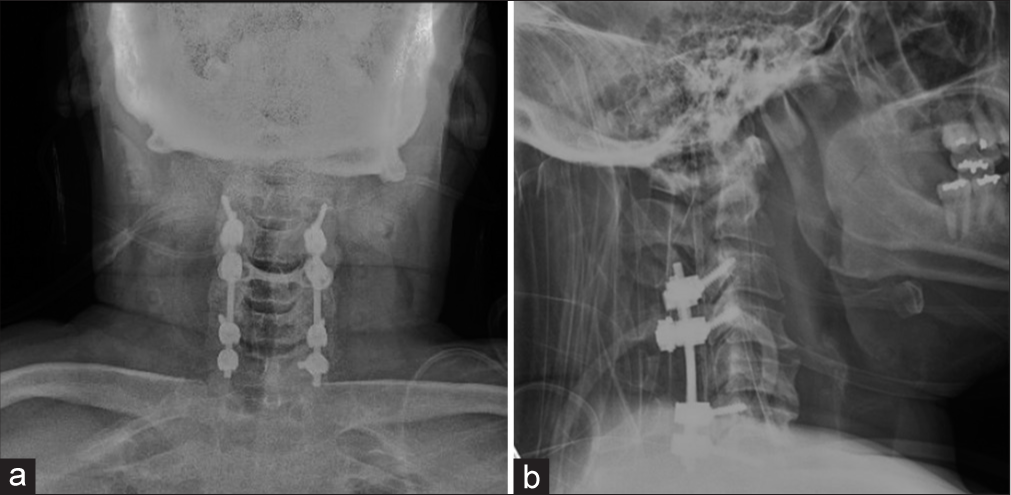
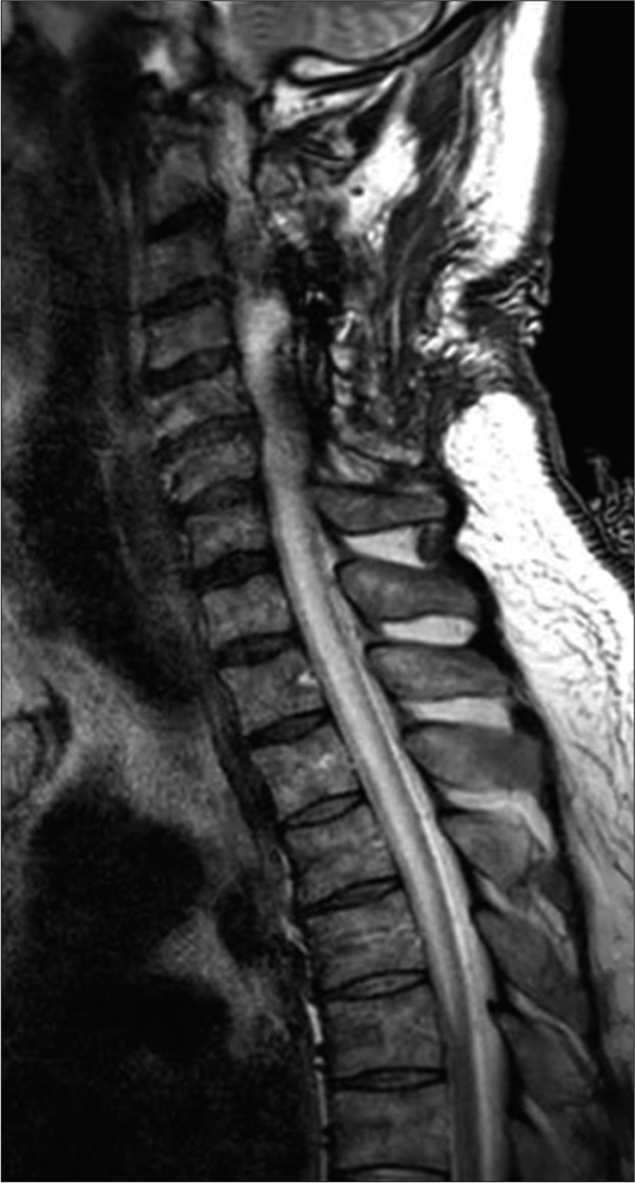
To decompress but not biopsy the C4–C6 intramedullary mass, the patient underwent a C3–C6 laminectomy and C3– C7 posterior spinal fusion. Although one day postoperatively, he developed a new right-sided motor hemiparesis that lasted for two weeks; the repeat MRI scan did not show any new cord lesions or increased intrinsic/extrinsic cord compression. Nine months later, the patient experienced four transient relapses of hemiparesis/quadriparesis (i.e., none of which warranted or were treated surgically due to no new MR findings) for which he received varying doses of rituximab, methotrexate, and corticosteroids [
DISCUSSION
Diagnosis of neurosarcoidosis and systemic sarcoidosis
The diagnosis of neurosarcoidosis is confirmed by laboratory evidence of central nervous system inflammation (i.e., elevated levels of CSF protein and/or cells) and MRI-documented neural lesions (i.e., T2 enhancement of meningeal or parenchymal tissues). In addition, patients may have evidence of systemic sarcoidosis and demonstrate cardiopulmonary, dermatologic, or ophthalmologic symptoms/signs and/or elevated inflammatory laboratory markers reflective of sarcoidosis requiring further medical management.[
No consensus regarding pharmaceutical management of neurosarcoidosis
There is presently no consensus regarding the pharmaceutical management of neurosarcoidosis. The first line of treatment is typically the administration of corticosteroids (i.e., varying dosage, duration, and method). However, if this fails, alternative immunomodulatory therapies are offered (i.e., tumor necrosis factor alpha inhibitors, infliximab). For instance, in Moravian and Segal’s series, seven patients with glucocorticoid-refractory neurosarcoidosis responded favorably to infliximab.[
Surgery
A decline in neurological function has been reported following extensive decompressive surgeries, underscoring the unpredictable course of neurosarcoidosis.[
CONCLUSION
Patients with neurosarcoidosis may respond unpredictably, with marked fluctuations/exacerbations and remissions, to either medical (i.e., primarily to corticosteroids and secondarily to immunotherapies) and/or surgical management (i.e., decompression with/without fusion).
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflict of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Chen HI, Lang SS, Coyne TM, Malhotra NR, Schuster JM. Intramedullary spinal sarcoidosis masquerading as cervical stenosis. World Neurosurg. 2013. 80: e375-80
2. Hayat GR, Walton TP, Smith KR, Martin DS, Manepalli AN. Solitary intramedullary neurosarcoidosis: Role of MRI in early detection. J Neuroimaging. 2001. 11: 67-70
3. Jefferson M. Sarcoidosis of the nervous system. Brain. 1957. 80: 540-56
4. Kasliwal MK, Harbhajanka A, Nag S, O’Toole JE. Isolated spinal neurosarcoidosis: An enigmatic intramedullary spinal cord pathology-case report and review of the literature. J Craniovertebral Junction Spine. 2013. 4: 76-81
5. Kwon DH, Lee SH, Kim ES, Eoh W. Intramedullary sarcoidosis presenting with delayed spinal cord swelling after cervical laminoplasty for compressive cervical myelopathy. J Korean Neurosurg Soc. 2014. 56: 436-40
6. Maroun FB, O’Dea FJ, Mathieson G, Fox G, Murray G, Jacob JC. Sarcoidosis presenting as an intramedullary spinal cord lesion. Can J Neurol Sci. 2001. 28: 163-6
7. Mathieson C, Mowle D, Ironside J, O’Riordan R. Isolated cervical intramedullary sarcoidosis--a histological surprise. Br J Neurosurg. 2004. 18: 632-5
8. McAllister BD, Rebholz BJ, Wang JC. Is posterior fusion necessary with laminectomy in the cervical spine?. Surg Neurol Int. 2012. 3: S225-31
9. Moravan M, Segal BM. Treatment of CNS sarcoidosis with infliximab and mycophenolate mofetil. Neurology. 2009. 72: 337-40
10. Nurboja B, Chaudhuri A, David KM, Casey AT, Choi D. Swelling and enhancement of the cervical spinal cord: When is a tumour not a tumour?. Br J Neurosurg. 2012. 26: 450-5
11. Oe K, Doita M, Miyamoto H, Kanda F, Kurosaka M, Sumi M. Is extensive cervical laminoplasty an effective treatment for spinal cord sarcoidosis combined with cervical spondylosis?. Eur Spine J. 2009. 18: 570-6
12. Pirau L, Lui F, editors. Neurosarcoidosis. StatPearls. Treasure Island, FL: StatPearls Publishing; 2023. p. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534768 [Last accessed on 2023 Jul 02]
13. Sakai Y, Matsuyama Y, Imagama S, Ito Z, Wakao N, Ishiguro N. Is decompressive surgery effective for spinal cord sarcoidosis accompanied with compressive cervical myelopathy?. Spine. 2010. 35: E1290-7
14. Saleh S, Saw C, Marzouk K, Sharma O. Sarcoidosis of the spinal cord: Literature review and report of eight cases. J Natl Med Assoc. 2006. 98: 965-76
15. Zajicek JP, Scolding NJ, Foster O, Rovaris M, Evanson J, Moseley IF. Central nervous system sarcoidosis--diagnosis and management. QJM. 1999. 92: 103-17