- Department of Neurosurgery, National Neuroscience Institute, Singapore.
DOI:10.25259/SNI_485_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yu Tung Lo, David Siu Kei Mak, Colum Patrick Nolan. Surgical management of vertebral metastatic gastrointestinal stromal tumor: Case illustration, literature review, and pooled analysis. 15-Oct-2020;11:343
How to cite this URL: Yu Tung Lo, David Siu Kei Mak, Colum Patrick Nolan. Surgical management of vertebral metastatic gastrointestinal stromal tumor: Case illustration, literature review, and pooled analysis. 15-Oct-2020;11:343. Available from: https://surgicalneurologyint.com/surgicalint-articles/10330/
Abstract
Background: Gastrointestinal stromal tumors (GISTs) very rarely metastasize to the vertebrae. Tyrosine kinase inhibitors (TKIs) confer favorable long-term survival and durable disease control for metastatic disease. Here, we reviewed a case and the literature to determine the various management options, and neurological outcomes for these patients.
Case Description: A 63-year-old Chinese female with metastatic jejunal GIST previously treated with various TKIs presented with the left lower limb weakness and a sensory level at T10. MRI revealed a T9 vertebral body tumor with cord compression. The tumor was excised and surgical fixation was performed. She received 30Gy of fractionated adjuvant radiotherapy. She achieved near-complete neurological recovery but died 2 months later from systemic disease progression.
Conclusion: Based on this case and a review of the literature, surgical intervention and treatment with TKIs with adjuvant RT can lead to comparable survival and neurological outcomes.
Keywords: Gastrointestinal stromal tumor, Spine metastasis, Spine surgery, Vertebral metastasis
INTRODUCTION
Metastasis of gastrointestinal stromal tumor (GIST) to the vertebral column is very rare; there are only a handful of reported cases in the literature. Tyrosine kinase inhibitors (TKIs) (e.g., imatinib mesylate) have significantly prolonged survival in metastatic GIST,[
Here, we report a case of metastatic vertebral GIST, and provide a summary of the current literature regarding treatment options, and long-term survival/neurological outcomes for these patients.
METHODS
Literature review
PubMed and MEDLINE were searched using the term “(GIST OR (gastrointestinal AND stromal)) AND (spine OR spinal OR vertebral)” on April 11, 2019. It yielded 47 results; Chinese, Japanese, and English articles were assessed. Patient characteristics were identified and summarized. Kaplan– Meier analysis was used to estimate the median overall survival, 1-year and 2-year survivals by pooling outcome data from individual case reports or series. Python version 3.7, and the lifelines package were used to perform survival analyses.[
CASE REPORT
History, examination, and imaging
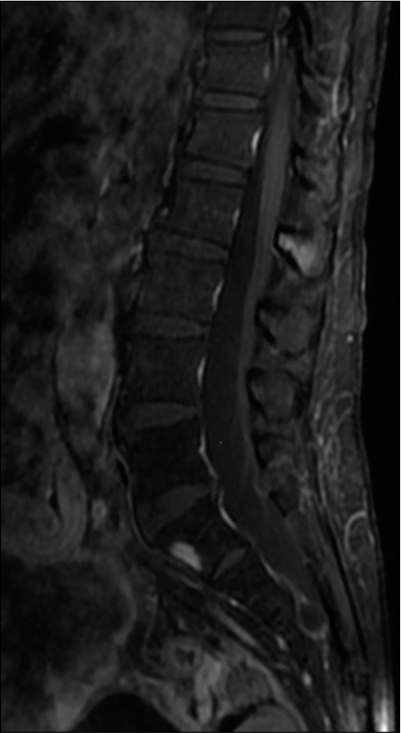
A 63-year-old Chinese female presented with 1-week history of progressive thoracolumbar back pain and left leg weakness with a T10 sensory level with preservation of sphincter function. She had been diagnosed with jejunal GIST 5 years previously and had known metastases to the liver, mesenteric lymph nodes, pelvis, and bladder. She had already received multiple lines of TKIs (imatinib, sunitinib, regorafenib, and dasatinib). The holospinal MR revealed a T9 enhancing lesion with an associated pathological fracture, spinal cord compression, and cord edema [
Operation
She underwent a T9 decompressive laminectomy for tumor excision and an instrumented fusion from T7 to T11; the diseased left T9 pedicle was excised. The tumor was gray, soft, friable, and hyper-vascular. The extradural component anterior to the cord was removed, and the cord was adequately decompressed.
Histopathology
The histopathology confirmed the diagnosis of metastatic GIST. It revealed lamellar bone fragments with nests and cords of epithelioid cells accompanied by moderate anisonucleosis, fair amounts of cytoplasm, and gland-like spaces in some areas. Background myxoid features were also noted. The tumor stained weakly for KIT (CD117), strongly for DOG-1, and negative for S-100, HMB45, desmin, CD31, ERG, Cam5.2, and AE1/3.
Postoperative course
Following surgery, she had no surgery-related complications, and nearly-completely recovered neurological function with a short rehabilitation stay. Adjuvant RT of 30 grays over ten fractions was administered from T7 to T11. Some residual back pain was noted and treated with oral analgesics. Postoperative X-rays demonstrated adequate location of instrumentation and preservation of alignment [
RESULTS
Patient demographics
From literature search, we identified nine patients (including this case) with metastatic vertebral GIST who were treated surgically. Four of these had neurological deficits: 2 motor, 1 sensory, and 1 malignant cord compression presenting with cauda equina syndrome [
There were “20 patients” managed non-surgically, whose baseline clinical characteristics were similar to the surgical patient population. Interestingly, they averaged 10 years older than the surgical patients (64 years old vs. 54 years old, respectively), and seven presented with motor/sensory deficits, while 11 had pain alone. There were no other statistically significant differences in baseline clinical characteristics between the surgical and non-surgical groups [
Pattern of vertebral metastasis
The lumbar (39%) and thoracic (39%) regions were the most common sites, followed by the sacrum (24%), and lastly, the cervical (15%) spine. Twenty (61%) of these patients also had bone metastases mainly involving the axial skeleton. In addition, 26 patients (79%) had visceral involvement, and 23 patients (70%) had liver metastasis. GIST tumors arose from a variety of locations: eleven from the small intestinal (33%), ten gastric (30%), six colorectal (18%), and six from extra-gastrointestinal sites [
Survival outcomes
The median overall survival of 27 patients, from time of diagnosis of vertebral GIST, was 34 months; 1-year survival was 74%, and 2-year survival was 56%. The survival curves for those managed surgically versus “non-surgically” did not differ significantly; in the surgical cohort, the median survival was 24 months, while in the nonsurgery cohort, it was 34 months [
Neurological outcomes
Rates of residual neurological deficits were comparable between the two groups [
DISCUSSION
Metastatic GIST
GISTs are rare mesenchymal tumors and constitute 0.1%–3% of all gastrointestinal neoplasms. They most commonly involve the stomach (60%), small intestine (15%), colon/ rectum (5%), and visceral organs (omentum) mesentery (5%).[
Genetics and chemotherapy
Most GISTs stain positive for KIT (CD117) and DOG-1. The identification of driver mutations in the KIT or PDGFRA (platelet-derived growth factor receptor alpha) genes led to the development of TKIs such as imatinib and sunitinib which significantly improved the survival of these patients.[
Treatment considerations
Most vertebral GIST metastases are symptomatic; 80% present with pain, and 38% with neurological deficits.
Comparison of all reported cases failed to show a significant difference in the median overall survival (24 vs. 34 months, P = 0.56) or neurological outcome (17% vs. 20% residual deficits, P = 0.87) between those managed surgically versus “non-surgically” (e.g., with TKIs and RT).
CONCLUSION
Metastatic GIST to the vertebral bodies is rare, and these patients have median survivals of 34 months. Successful treatment may include surgical and nonsurgical (TKIs and RT) options.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aktan M, Koc M, Yavuz BB, Kanyilmaz G. Two cases of gastrointestinal stromal tumor of the small intestine with liver and bone metastasis. Ann Transl Med. 2015. 3: 259
2. Balachandran VP, DeMatteo RP. Gastrointestinal stromal tumors: Who should get imatinib and for how long?. Adv Surg. 2014. 48: 165-83
3. Barrière J, Thariat J, Vandenbos F, Bondiau PY, Peyrottes I, Peyrade F. Diplopia as the first symptom of an aggressive metastatic rectal stromal tumor. Onkologie. 2009. 32: 345-7
4. Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA. Long-term results from a randomized phase II trial of standard-versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008. 26: 620-5
5. Bor-Ren H, Hsien-Chih C, Tai-Ngar L, Wen-Ching T. Epidural metastases from a gastrointestinal stromal tumour. J Clin Neurosci. 2008. 15: 82-4
6. Chang SW, Cho W, Chang UK. Treatment for spinal metastasis of gastrointestinal stromal tumors: Two case reports. Nerve. 2018. 4: 97-100
7. Chou SQ, Tse KS, Wong WK, Chan SC. Vulval gastrointestinal stromal tumours with bone metastases. J Hong Kong Coll Radiol. 2010. 13: 88-90
8. Chu TY, Wong CS. Bone metastases from gastrointestinal stromal tumour: Correlation with positron emission tomography-computed tomography. J HK Coll Radiol. 2009. 117: 172-5
9. Davidson-Pilon C, Kalderstam J, Zivich P, Kuhn B, FioreGartland A, Moneda L.editors. Cam Davidson Pilon/Lifelines: V0.20.4. 2019. p.
10. Demetri GD, von Mehren M, Blanke CD, van den Abbeele AD, Eisenberg B, Roberts PJ. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002. 347: 472-80
11. di Scioscio V, Greco L, Pallotti MC, Pantaleo MA, Maleddu A, Nannini M. Three cases of bone metastases in patients with gastrointestinal stromal tumors. Rare Tumors. 2011. 3: 51-3
12. Din OS, Wool PJ. Treatment of gastrointestinal stromal tumor: Focus on imatinib mesylate. Ther Clin Risk Manag. 2008. 4: 149-62
13. Feki J, Bouzguenda R, Ayedi L, Bradi M, Boudawara T, Daoud J. Bone metastases from gastrointestinal stromal tumor: A case report. Case Rep Oncol Med. 2012. 2012: 1-3
14. Fujisawa T, Matsumoto Y, Nishizawa A, Takata M. A case of liver and bone metastases after complete resection of gastric GIST effectively treated with radiotherapy and imatinib mesylate. Nihon Shokakibyo Gakkai Zasshi. 2013. 110: 1258-64
15. Gong L, Li YH, Zhao HD, Zhao JY, Zhang W. The clinicopathologic observation, c-KIT gene mutation and clonal status of gastrointestinal stromal tumor in the sacrum. BMC Gastroenterol. 2009. 9: 43
16. Ishi Y, Nakayama N, Kobayashi H, Yamaguchi S, Terasaka S, Houkin K. Successful removal of a metastatic gastrointestinal stromal tumor in the craniovertebral junction using an occipital artery to posterior inferior cerebellar artery bypass. Case Rep Neurol. 2014. 6: 139-43
17. Ishikawa A, Teratani T, Ono S, Ochiai T, Kakinoki N, Kishimoto Y. A case of gastrointestinal stromal tumor with liver and bone metastases effectively treated with radiofrequency ablation and imatinib mesylate. Nihon Shokakibyo Gakkai Zasshi. 2006. 103: 1274-9
18. Jain A, Mangaladevi B, Halanaik D, Dubashi B, Chandra S. Mesentric gastrointestinal stromal tumor with bone metastases. Indian J Cancer. 2011. 48: 383
19. Jati A, Tatli S, Morgan JA, Glickman JN, Demetri GD, Van den Abbele A. Imaging features of bone metastases in patients with gastrointestinal stromal tumors. Diagn Interv Radiol. 2012. 18: 391-6
20. Kaku S, Tanaka T, Ohtuka T, Seki K, Sawauchi S, Numoto RT. Perisacral gastrointestinal stromal tumor with intracranial metastasis. Case report. Neurol Med Chir (Tokyo). 2006. 46: 254-7
21. Nakajima T, Miwa S, Ando T, Yamada K, Miyazaki T, Hosokawa A. Three cases of gastrointestinal stromal tumor (GIST) with bone metastasis. Nihon Shokakibyo Gakkai Zasshi. 2008. 105: 836-40
22. Patrikidou A, Domont J, Chabaud S, Ray-Coquard I, Coindre JM, Bui-Nguyen B. Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR14 trial of the French Sarcoma Group. Eur J Cancer. 2016. 52: 173-80
23. Rochigneux P, Mescam-Mancini L, Perrot D, Bories E, Moureau-Zabotto L, Sarran A. Gastrointestinal stromal tumour with synchronous bone metastases: A case report and literature review. Case Rep Oncol. 2017. 10: 66-76
24. Sahin E, Yetişyiğit T, Oznur M, Elboğa U. Gastric gastrointestinal stromal tumor with bone metastases-case report and review of the literature. Klin Onkol. 2014. 27: 56-9
25. Shimizu T, Murakami H, Sangsin A, Demura S, Kato S, Shinmura K. En bloc corpectomy for late gastrointestinal stromal tumor metastasis: A case report and review of the literature. J Med Case Rep. 2018. 12: 300
26. Slimack NP, Liu JC, Koski T, McClendon J, O’Shaughnessy BA. Metastatic gastrointestinal stromal tumor to the thoracic and lumbar spine: First reported case and surgical treatment. Spine J. 2012. 12: e7-12
27. Takeda Y, Nakahira S, Katsura Y, Ohmura Y, Kusama H, Kuroda Y. A case of recurrent duodenal gastrointestinal stromal tumor resistant to imatinib and sunitinib, successfully treated with regorafenib. Gan To Kagaku Ryoho. 2014. 41: 1545-7
28. von Mehren M, Joensuu H. Gastrointestinal stromal tumors. J Clin Oncol. 2018. 36: 136-43
29. Wang ZQ, Wang S, Ye YJ, Kang YL, Sun KK, Zheng HF. Gastrointestinal mesenchymal tumors: A clinical pathologic and immunohistochemical study of 210 cases. Zhonghua Wei Chang Wai Ke Za Zhi. 2007. 10: 11-6
30. Waterman BR, Kusnezov N, Dunn JC, Hakim MN. Aggressive gastrointestinal stromal tumor with spinal metastases: A case report. Mil Med. 2015. 180: e618-21
31. Zhang Y, Zhang A, Song L, Li X, Zhang W. Primary extragastrointestinal stromal tumor on FDG PET/CT. Clin Nucl Med. 2018. 43: 702-3