- Department of Pediatric Neurosurgery, Federal Center of Neurosurgery, Novosibirsk, Russian Federation,
- Research Institute of Clinical and Experimental Lymphology - Branch of the Federal State Budgetary Scientific Institution “Federal Research Center Institute of Cytology and Genetics of the Siberian Branch of the Russian Academy of Sciences”, Novosibirsk, Russia,
- Department of Functional Diagnostics, Federal Center of Neurosurgery, Russian Federation
- Department of Rehabilitation Medicine, Federal Center of Neurosurgery, Novosibirsk, Russian Federation.
Correspondence Address:
Anton Olegovich Ivanov, Department of Pediatric Neurosurgery, Federal Center of Neurosurgery, Novosibirsk, Russian Federation.
DOI:10.25259/SNI_478_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sergey Afanasievich Kim1,2, Anton Olegovich Ivanov1, Anastasia Anatolievna Halepa3, Anna Alexeevna Sysoeva1, Galina Alexandrovna Gunenko4. Surgical treatment of epilepsy in a patient with bilateral periventricular nodular heterotopia: A case report. 11-Aug-2023;14:283
How to cite this URL: Sergey Afanasievich Kim1,2, Anton Olegovich Ivanov1, Anastasia Anatolievna Halepa3, Anna Alexeevna Sysoeva1, Galina Alexandrovna Gunenko4. Surgical treatment of epilepsy in a patient with bilateral periventricular nodular heterotopia: A case report. 11-Aug-2023;14:283. Available from: https://surgicalneurologyint.com/surgicalint-articles/12497/
Abstract
Background: Periventricular nodular heterotopia (PNH) is a rare pathological condition characterized by the presence of nodules of gray matter located along the lateral ventricles of the brain. The condition typically presents with seizures and other neurological symptoms, and various methods of surgical treatment and postoperative outcomes have been described in the literature.
Case Description: We present a case study of a 17-year-old patient who has been experiencing seizures since the age of 13. The patient reported episodes of loss of consciousness and periodic freezing with preservation of posture. Two years later, the patient experienced his first generalized tonic-clonic seizure during nocturnal sleep and was subsequently admitted to a neurological department. A magnetic resonance imaging scan of the brain with an epilepsy protocol (3 Tesla) confirmed the presence of an extended bilateral subependymal nodular heterotopy at the level of the temporal and occipital horns of the lateral ventricles, which was larger on the left side, and a focal subcortical heterotopy of the right cerebellar hemisphere. The patient underwent a posterior quadrant disconnection surgery, which aimed to isolate the extensive epileptogenic zone in the left temporal, parietal, and occipital lobes using standard techniques. As of today, 6 months have passed since the surgery and there have been no registered epileptic seizures during this period following the surgical treatment.
Conclusion: Although PNHs can be extensive and located bilaterally, surgical intervention may still be an effective way to achieve seizure control in selected cases.
Keywords: Case report, Epilepsy, Periventricular nodular heterotopia, Posterior quadrant disconnection, Seizure, Surgical treatment
INTRODUCTION
Periventricular nodular heterotopia (PNH) is a cerebral malformation that occurs due to impaired neuronal migration. It is characterized by clusters of heterotopic tissue in the form of rounded or irregularly shaped nodules separated by layers of myelin. These nodules can be found either lining the walls of the lateral ventricles or protruding into their cavity. They may be unilateral or bilateral, small or large, and adjacent or nonadjacent. The main clinical manifestation of this condition is epileptic seizures, which are usually resistant to pharmacological treatment. Treatment of epilepsy in patients with PNH involves surgical intervention, but choosing the appropriate surgical approach can be challenging. In this article, we describe a clinical case of a patient with bilateral periventricular heterotopia.
CLINICAL CASE
Patient P, a 17 years old, presented with a history of seizures since the age of 13. The episodes involved loss of consciousness, periodic freezing with preservation of posture. Two years later, during nocturnal sleep, Patient P experienced a generalized clonic-tonic seizure for the first time and was hospitalized in a neurological department. An electroencephalography (EEG) was conducted and it showed sharp-slow wave complexes in the left occipital region with diffuse propagation. Brain magnetic resonance imaging (MRI) revealed heterotopia of the gray matter of the left hemisphere and nodules of the lateral ventricles. As a result, anticonvulsive therapy was prescribed, consisting of 500 mg of valproic acid twice a day. Subsequently, topiramate was added to the treatment. Despite receiving treatment, the patient continued to experience frequent seizures (up to three per week). However, after being prescribed carbamazepine, the frequency of seizures decreased and topiramate was discontinued. The patient’s seizure symptoms included stopping activity, turning his head to the right, automatisms in the hands, and swallowing movements. The seizures typically lasted up to 2 min and occurred once every 10–14 days. Due to the persistence of seizures and the presence of structural changes in the brain, the epileptologist recommended surgical treatment for the patient’s epilepsy. After consultation with a neurosurgeon, the patient was referred for a presurgical examination.
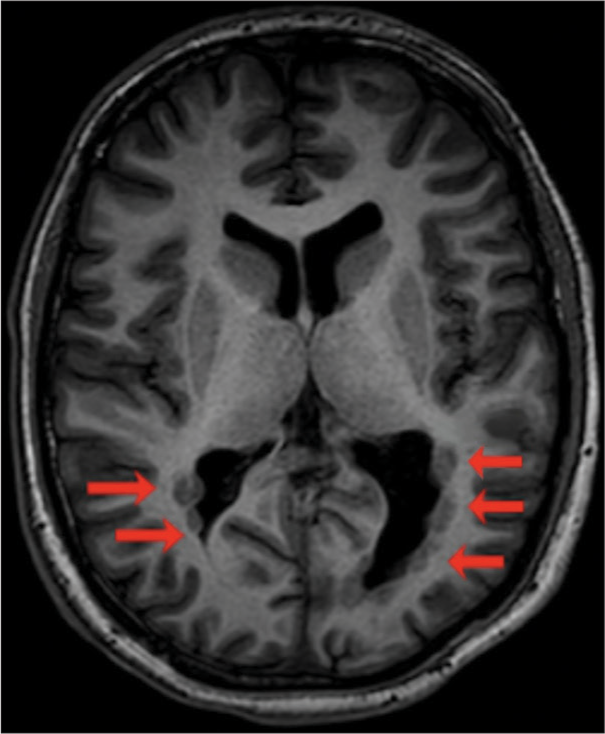
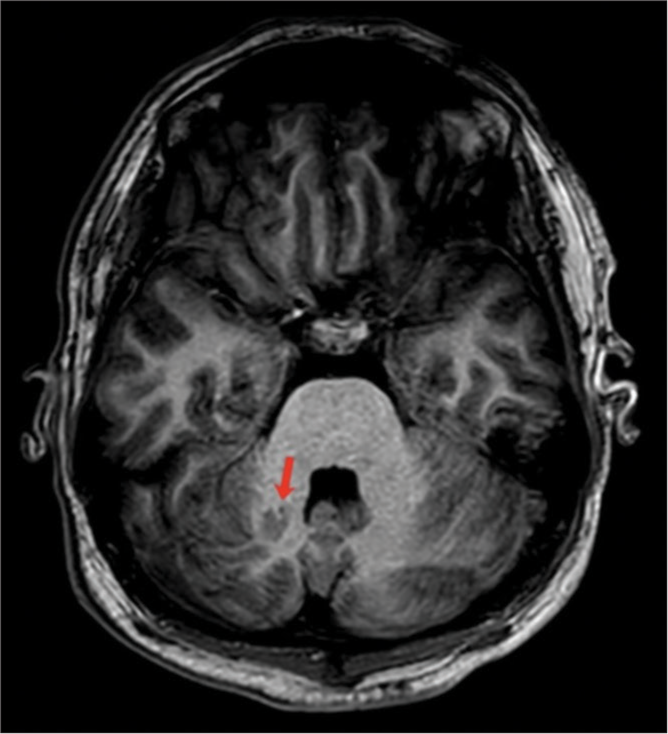
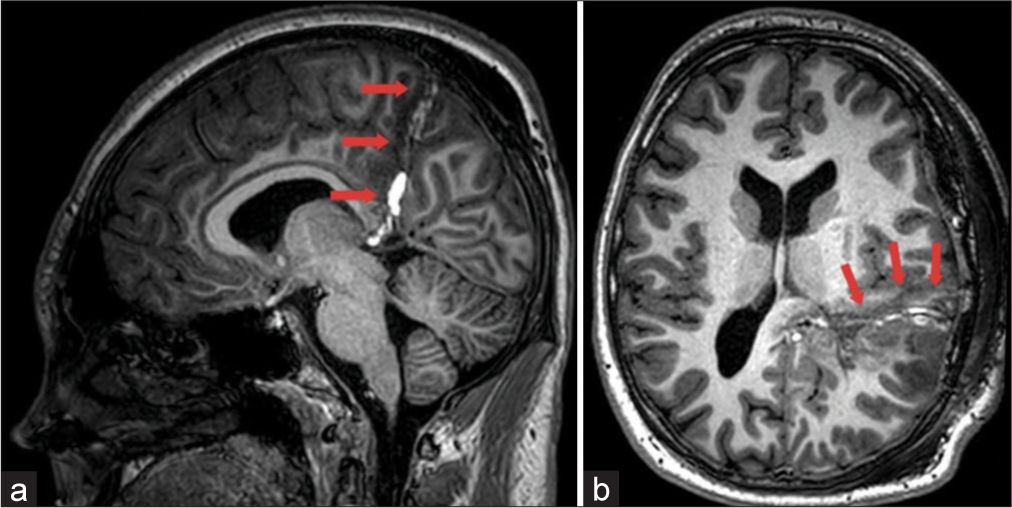
The child was given valproic acid 500 mg twice a day and carbamazepine 400 mg twice a day on admission. There were no focal symptoms detected during the neurological examination. An MRI of the brain with epilepsy protocol (3 Tesla) confirmed the presence of an extended bilateral subependymal nodular heterotopy at the level of the temporal and occipital horns of the lateral ventricles. The heterotopy was larger on the left side [
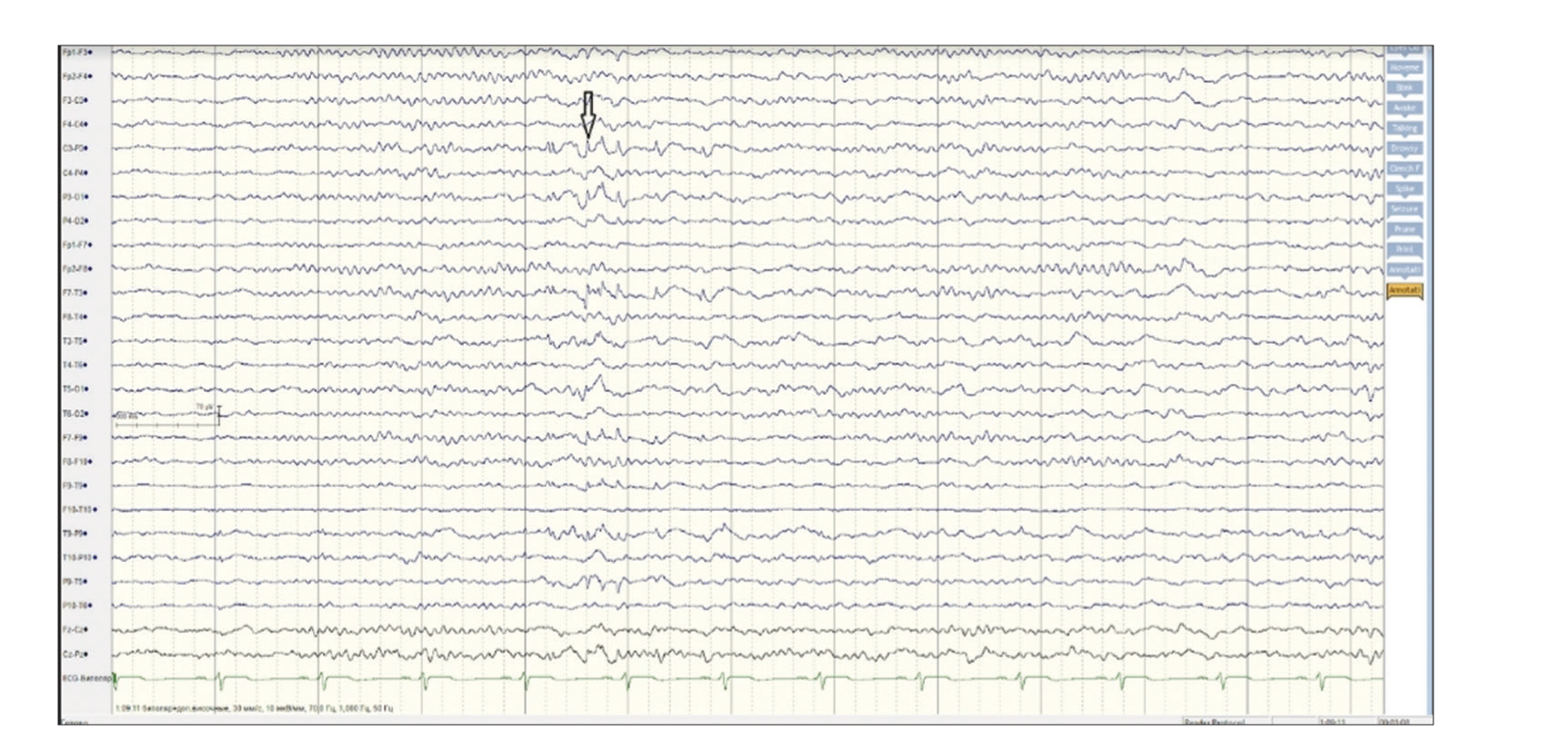
During the study, a focal motor seizure was recorded. It began during sleep, and then the person woke up and turned to the right. He withdrew his right arm, had inarticulate speech, and experienced faciobrachial clonies. Then, he rolled over onto his stomach and froze before falling back asleep.
On the EEG, before clinical onset, there was a discharge of fast isolated activity in C3-P3-T3-T5-T9-P9, followed by a diffuse electrodecrement, and rhythmic left hemispheric activity with emphasis in the parietoposterior temporal area (P3-T5-T9). The EEG onset zone was identified as the left centro-parietal-temporal region (P3-P3-T3-T5-T9-P9).
Due to the extensive anatomical lesion and widespread ictal area, it was decided to proceed with invasive video-EEG monitoring. To accomplish this, depth electrodes were surgically implanted in the left temporo-parieto-occipital area. The localization of the depth electrodes for invasive EEG-monitoring stereo electroencephalography (SEEG) is found in
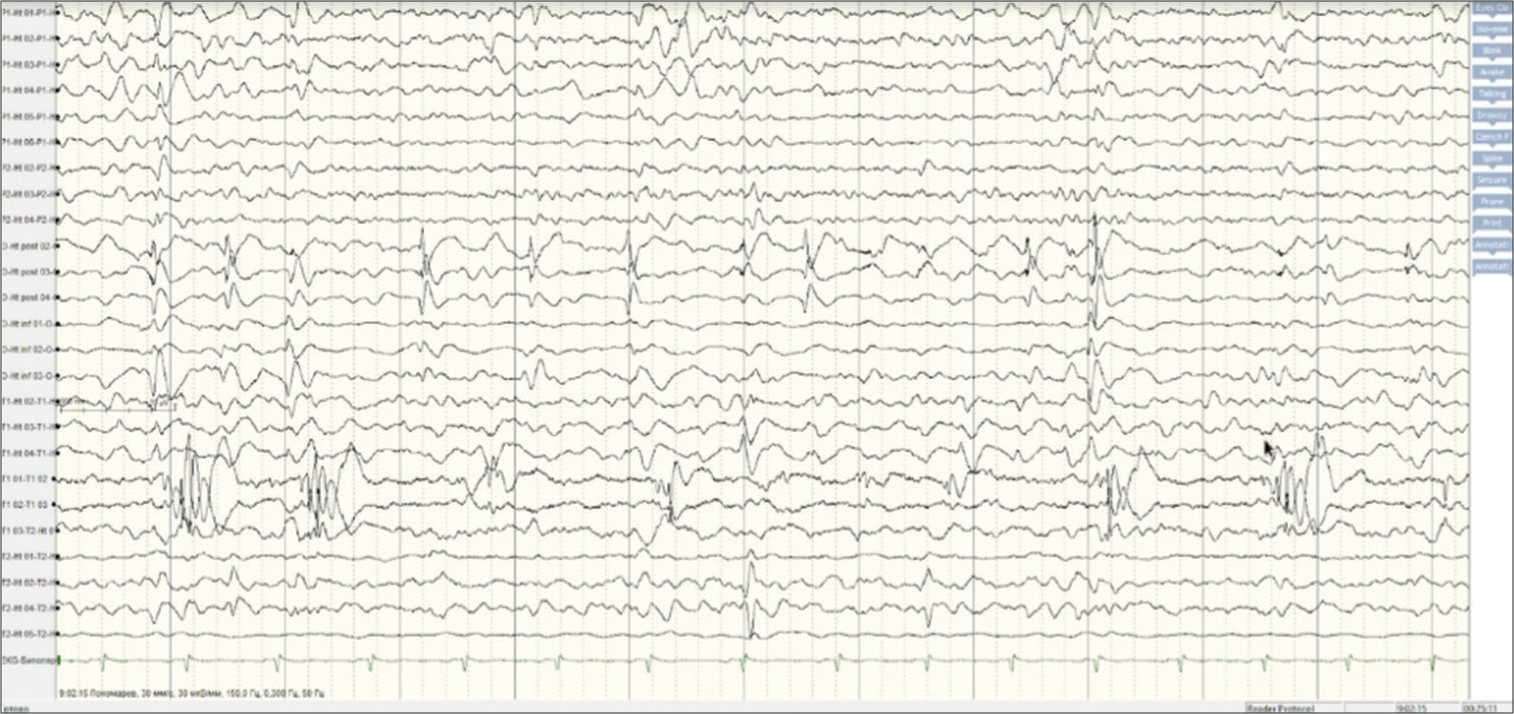
The EEG was recorded for 6 days using the implanted electrodes [
Figure 5:
Invasive electroencephalography (EEG), 12 sec intracranial EEG page (noisy and isoelectric channels excluded for clarity), bipolar montage, passband of 0, 3–150 Hz with a gain of 30 µV/ mm. Interictal synchronous spikes in the Occipital -heterotopia posterior (O-Ht post), Occipital -heterotopia inferior (O-Ht inf) electrodes as well as in the T1 electrode were observed.
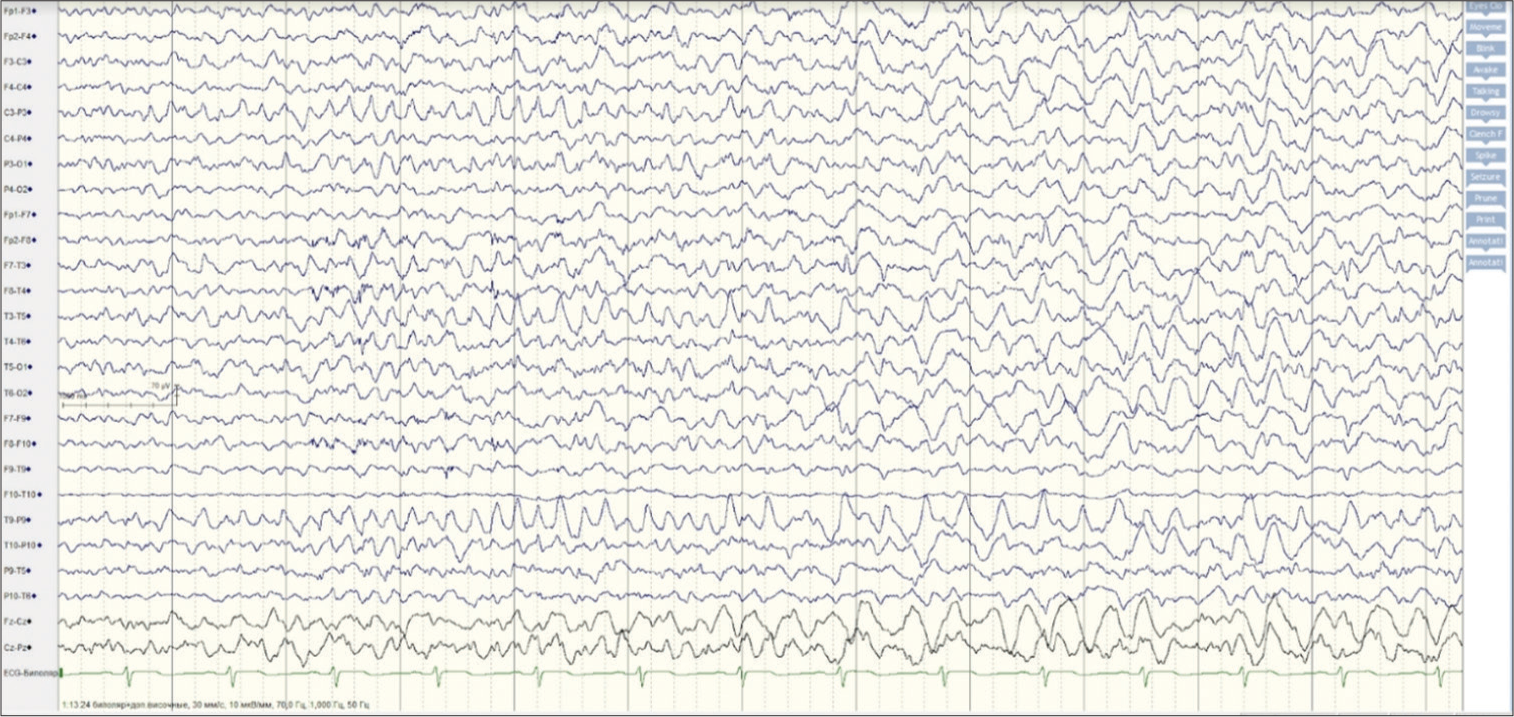
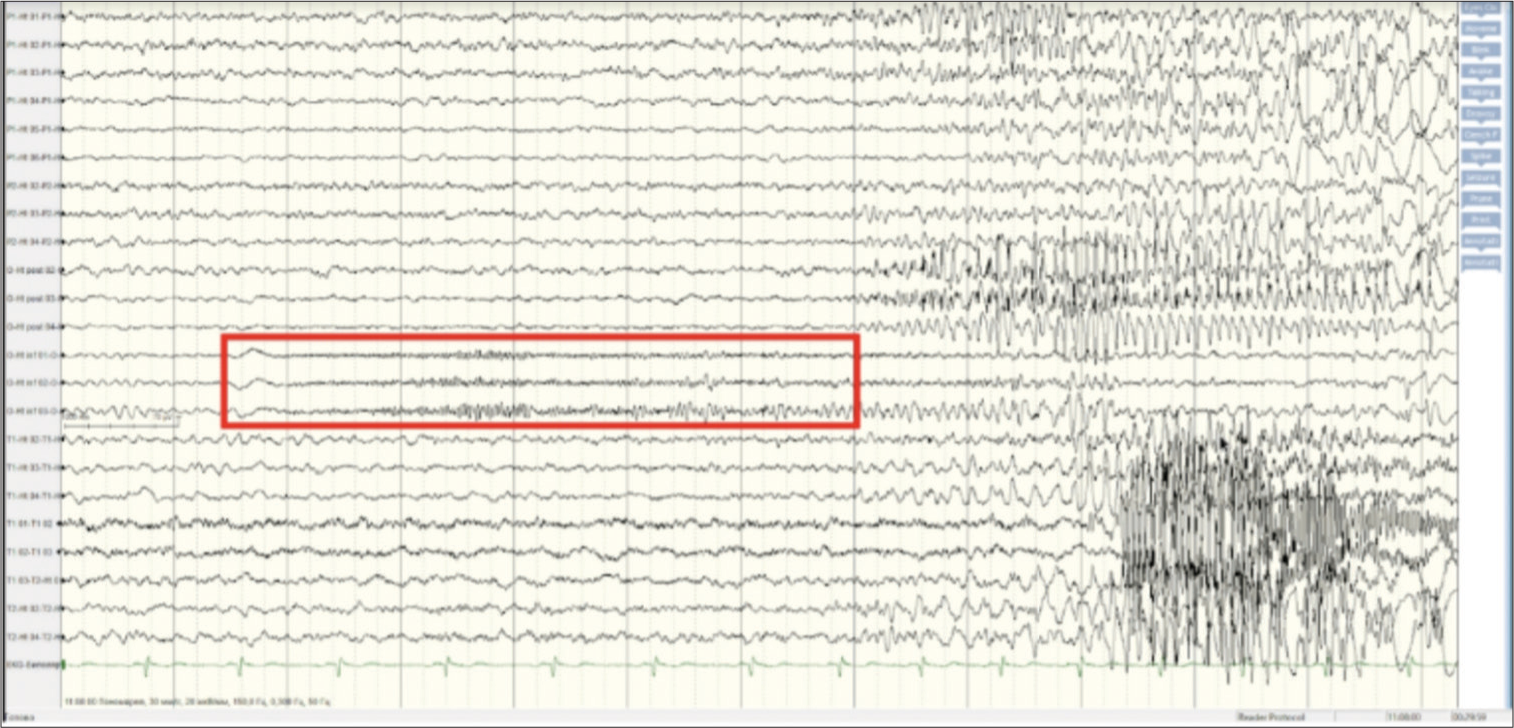
During the study, 20 focal motor seizures were recorded, two of which progressed into bilateral tonic-clonic seizures. Most seizures were brief and went unnoticed by both the patient and the caregiver. They typically occurred during wakefulness, with the patient opening his eyes, blinking frequently, looking to the right, and sometimes turning his head to the right. On SEEG, there was a focal electrodecrement, followed by low amplitude fast activity (LAFA) in the O-Ht inf (2, 3, 4) contacts, and rhythmic island-wave and peak-wave activity in the O-Ht post (2–4) and P1-Ht (1–3) contacts, sometimes with a wider spread.
The patient experienced two focal seizures, which developed into tonic-clonic seizures. Both seizures began during wakefulness and involved pronounced movements to the right side of the body. The seizures then progressed to orofacial and faciobrachial clonic movements to the right, followed by bilateral tonic-clonic seizures. In addition, three focal motor faciobrachial seizures to the right were observed.
During SEEG, a focal electrodecrement was noted, followed by LAFA in the O-Ht inf leads (2, 3, 4), and rhythmic isolated peak-wave activity in the O-Ht post contacts (2–4) and P1-Ht contacts (1–3). This activity then spread widely, with initial activity observed in the O-Ht inf contacts (2, 3, 4).
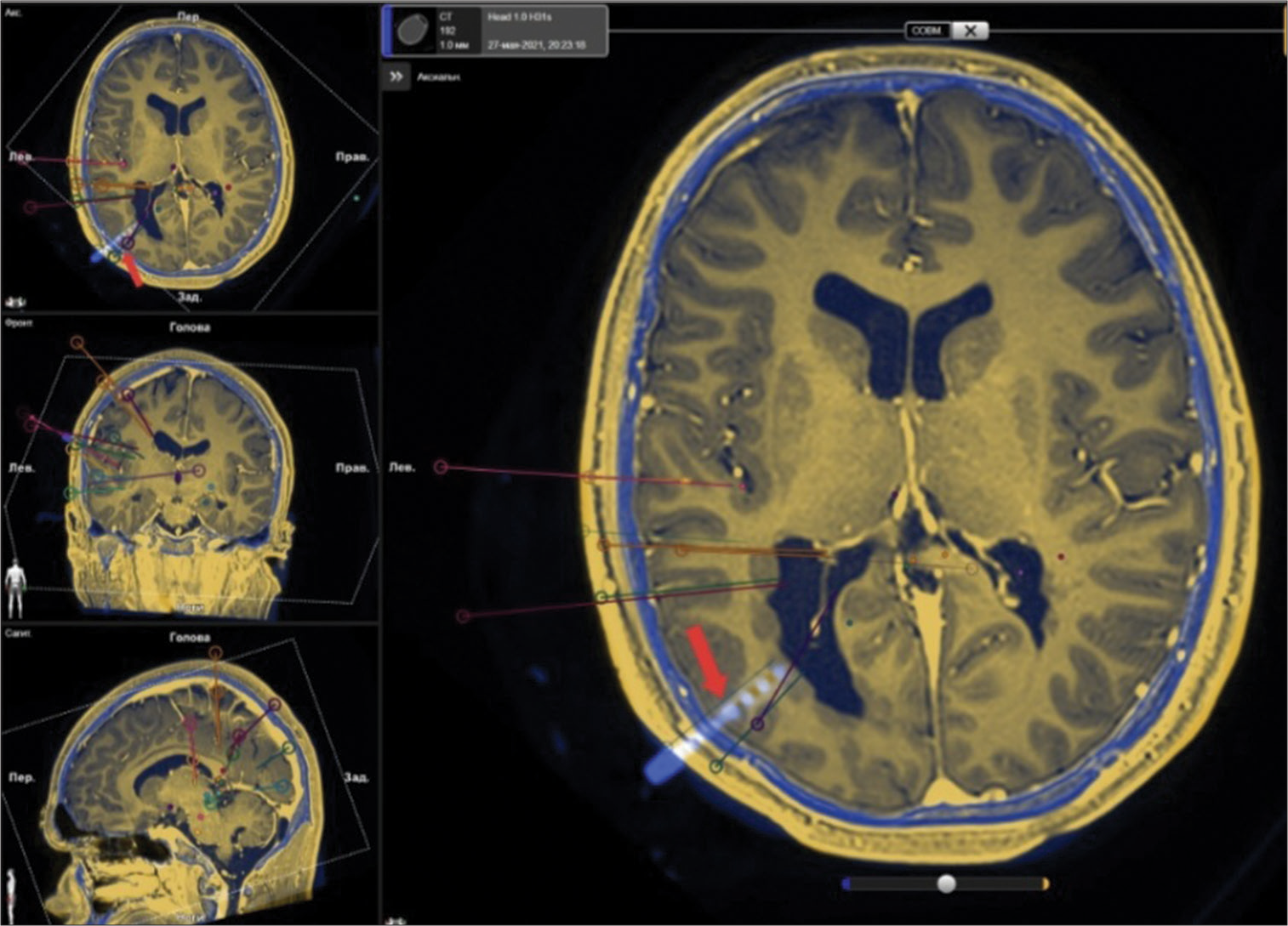
The location of the implanted depth electrodes was visualized through the fusion of MRI and computed tomography scans of the patient [
Figure 7:
A fusion of magnetic resonance imaging and computed tomography scans of the patient who underwent implantation of depth electrodes. The planned trajectories for electrode placement are represented by colored straight lines. In addition, one of the implanted electrodes is visible and indicated by the red arrow. Yellow - soft tissues and brain. Blue - skull bones.
After the examination, the patient’s case was reviewed during the epilepsy conference. Based on the SEEG results, an extensive epileptogenic zone was identified with registration of interictal activity from electrodes located in the left temporal, parietal, and occipital lobes, as well as initial activity in the occipital area. Epileptiform activity was detected from contacts located both directly in the thickness of the heterotopia and in the localized cortex. Due to the extent of the heterotopia (including the temporal horn, atrium, and posterior parts of the body of the lateral ventricle) and the involvement of the neocortex in epileptogenesis, the effectiveness of isolated destruction of intraventricular nodules was considered uncertain. Therefore, it was decided that it would be advisable to deactivate the entire epileptogenic zone.
Preoperative assessment of speech functions
The general mental status assessment revealed that the patient exhibited adequate behavior during preoperative examination of speech functions. The patient was able to communicate well with the speech therapist, demonstrated correct orientation in the environment and current situation, answered all questions, followed instructions, and reacted appropriately to success and failure with adequate emotional responses. The patient had no complaints and did not exhibit a reduced level of mental activity. The patient displayed good task engagement, stable voluntary attention, and work capacity. The patient was able to work productively for a period exceeding 45 min. Regarding education, the patient was a 10th-grade student in a secondary general school before the onset of the current medical condition. The patient’s academic performance was satisfactory and no speech disorders such as aphasia or dysarthria were detected in the status.[
To determine the dominance of the right hemisphere in speech function, functional MRI was performed, taking into account the localization of the epileptogenic zone in the left hemisphere. During the assessment of active word pronunciation (Broca’s area), asymmetric activation was detected in the posterior third of the right inferior frontal gyrus and symmetric activation was observed in the precentral gyrus in the motor representation zone of the face. For passive listening (Wernicke’s area), asymmetric activation was found in the posterior third of the right superior temporal gyrus, while symmetric activation was observed in the projection of the primary auditory cortex on both the left and right sides. Based on these findings, it was determined that the right hemisphere dominated in speech function.
However, due to the extent of the proposed intervention, it was deemed necessary to conduct the Wade test to more reliably assess the dominance of the hemispheres in relation to speech function. The results of the study showed that the introduction of an anesthetic agent (propofol 10 mg) into the left internal carotid artery (ICA) had no effect on speech function. On the other hand, the introduction of an anesthetic agent (propofol 10 mg) into the right ICA resulted in a pronounced suppression of speech function. Based on the results of the Wada test, the presumed lateralization of speech was in the right hemisphere.
The patient underwent posterior quadrant disconnection (PQD) surgery, which aimed to isolate the extensive epileptogenic zone of the left temporal, parietal, and occipital lobes [
The patient underwent a postoperative assessment of speech functions. The general mental status of the patient was found to be adequate and he was able to communicate well with the speech therapist. The patient displayed correct orientation in their environment and situation, answered all questions, followed instructions, and reacted appropriately to success and failure with adequate emotional reactions. The patient did not report any active complaints. His level of mental activity was average, and he engaged well in the task. However, mild fluctuations of voluntary attention and work capacity were observed against the background of fatigue. After the surgery, the patient experienced speech impairments of amnestic aphasia type, which was of a mild degree of severity, as revealed by a standardized comprehensive assessment tool called “Russian Aphasia Test.”[
The patient was discharged after 13 days of hospitalization, and as of today, which marks the 6-month follow-up period, no epileptic seizures have been registered after the surgical treatment. The patient is still taking anticonvulsants.
DISCUSSION
PNH is a rare pathological condition. The literature only contains descriptions of isolated clinical observations, without any data on its frequency of occurrence.
The etiology of this condition is closely related to a mutation in the FLNA gene, which is more commonly found in women. This mutation results in a loss of function in the FLNA gene, leading to neuronal migration disorders. A less common cause is a mutation in the ARFGEF2 gene.[
The classic presentation of the FLNA gene mutation includes bilateral periventricular heterotopic gray matter nodules, which are mainly concentrated around the anterior horns and bodies of the lateral ventricles. In addition, dysplasia of the corpus callosum or hippocampus, cerebellar hypoplasia, polymicrogyria, and microcephaly may also be observed.[
PNH usually manifests with epileptic seizures, which are often focal and drug-resistant, and starts between the ages of 2 and 14 years.[
Periventricular heterotopia can be classified according to the location of the nodes, which can be bilateral and symmetrical, bilateral with single nodes, bilateral and asymmetrical, unilateral, or unilateral with spreading into the cortex.[
Studies have shown that there are differences in clinical manifestations and outcomes depending on the form of periventricular heterotopia.[
Diagnostics
The primary diagnostic method is brain MRI, which can identify the presence of periventricular accumulations of gray matter.[
Brain computed tomography can also be used to reveal nodules isodense in relation to the gray matter. These nodules are diffusely lining the lateral ventricles, which gives them an irregular and festooned appearance. However, these periventricular nodules do not accumulate contrast agent.[
One of the most important methods of examination for patients with pharmacoresistant epilepsy is 24-h scalp video-EEG monitoring of the ictal and interictal periods. This method can help differentiate epileptic seizures from nonepileptic ones, classify seizures, and localize the seizure onset zone.[
Invasive examination is necessary to confirm the involvement of heterotopia in epileptogenesis, determine the dominant focus in a bilateral location, and clarify the degree of involvement of other anatomical structures. In addition, implanted depth electrodes can be used for further radiofrequency thermocoagulation.[
Observations have shown that seizures originating from the neocortex and hippocampus have been registered using intracranial depth electrodes, indicating the possibility that other anatomical structures may cause epileptic discharges in patients with bilateral periventricular nodal heterotopia.[
It is also important to note that we cannot rule out the possibility that bilateral periventricular nodal heterotopia may be the only source of epileptogenic activity.
Treatment
When drug treatment is ineffective in controlling epileptic seizures, surgical treatment becomes necessary. One option for surgical intervention is stereotactic destruction of small heterotopic nodes.[
Stereotactic radiosurgery (SRS)[
MRg-FUS is a minimally invasive technique that was originally approved for the therapy of essential tremor. However, it is currently considered less suitable for PNH ablation, as the accuracy and extent of the lesion are inferior to other surgical methods. In fact, the error in this method can be as much as 3 cm.[
Another method of surgical treatment involves the removal of a heterotopic focus with local resection of the normotopic part of the cerebral cortex.[
In cases where there is extended or bilateral periventricular heterotopia combined with the involvement of cortical structures in epileptogenesis, minimally invasive destruction of nodules or selective removal may not be sufficient. More extensive resections may be necessary.
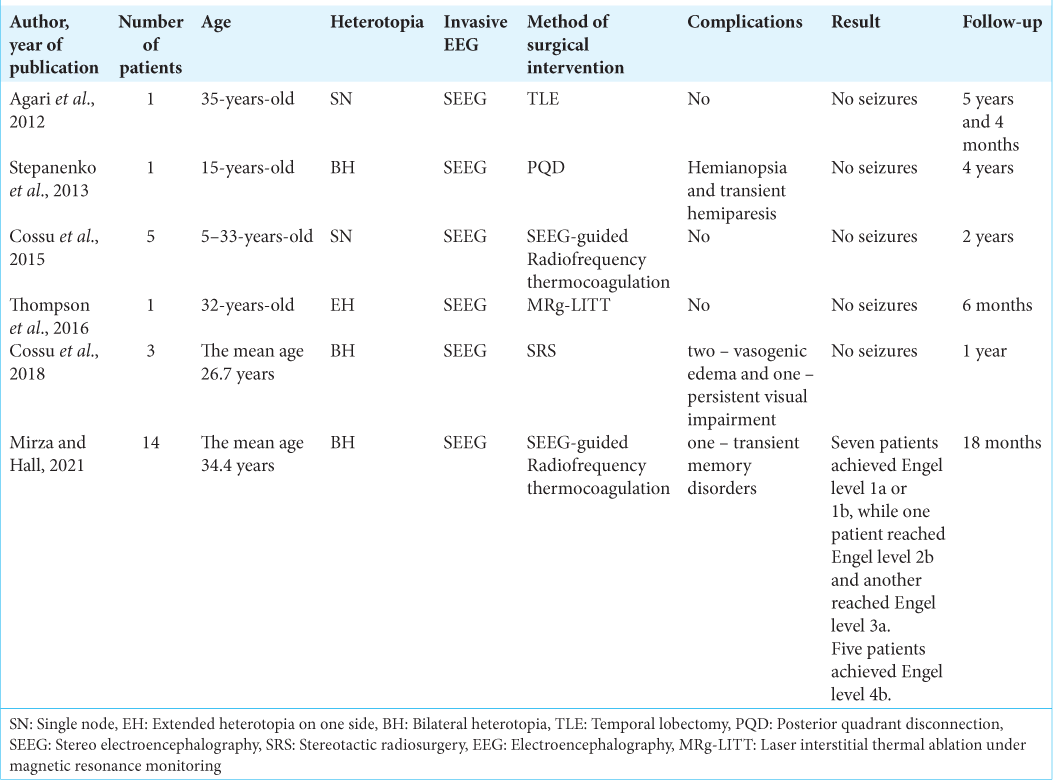
The publication by Stepanenko et al. in 2013 describes the case history of a 15-year-old patient suffering from pharmacoresistant epilepsy. The patient had bilateral PNH, combined with cortical dysplasia in the right temporoparietal region, while the left hemisphere showed no signs of cortical dysplasia. The invasive monitoring revealed rhythmic theta-delta activity during the interictal period and rapid activity at the beginning of the ictal pattern in the right temporal and parietal regions. The surgical procedure included an anterior temporal lobectomy, removal of the right heterotopic node, dissection of the posterior corpus callosum, and isolation of the temporoparieto-occipital complex by dissection behind the sensorimotor.[
Here, we presented a case study of a patient with bilateral extended subependymal nodular heterotopia located at the level of the temporal and occipital horns of the lateral ventricles of the brain. The patient’s condition was clinically manifested by focal motor seizures that evolved into bilateral tonic-clonic seizures.
Before the surgical intervention, the patient underwent a presurgical examination, including SEEG, which revealed an extensive epileptogenic zone in the left temporal, parietal, and occipital lobes. Due to the extent of the heterotopia (temporal horn, atrium, and posterior parts of the body of the lateral ventricle) as well as the involvement of the neocortex in epileptogenesis, surgical intervention was performed. The aim of the surgery was to isolate the extensive epileptogenic zone of the left temporoparietal-occipital area.
The surgical intervention, in the form of PQD, did not result in deterioration of the patient’s neurological status, except for the predicted hemianopsia, and allowed for the achievement of seizure control.
CONCLUSION
Bilateral PNH is a rare genetic pathology that commonly leads to epileptic seizures that are resistant to drug therapy. Despite the bilateral location and extent of the heterotopias, surgical intervention in selected cases can be an effective method to control seizures.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Agari T, Mihara T, Baba K, Kobayashi K, Usui N, Terada K. Successful treatment of epilepsy by resection of periventricular nodular heterotopia. Acta Med Okayama. 2012. 66: 487-92
2. Akkol S, Kucyi A, Hu W, Zhao B, Zhang C, Sava-Segal C. Intracranial electroencephalography reveals selective responses to cognitive stimuli in the periventricular heterotopias. J Neurosci. 2021. 41: 3870-8
3. Angulo-Maldonado M, Lara-Sarabia O, Cadena-Bonfanti A, Ulloa-Piza A. X-linked hereditary periventricular nodular heterotopia. Neurología (Engl Ed). 2022. 37: 232-4
4. Battaglia G, Franceschetti S, Chiapparini L, Freri E, Bassanini S, Giavazzi A. Electroencephalographic recordings of focal seizures in patients affected by periventricular nodular heterotopia: Role of the heterotopic nodules in the genesis of epileptic discharges. J Child Neurol. 2005. 20: 369-77
5. Boulogne S, Pizzo F, Chatard B, Roehri N, Catenoix H, Ostrowsky-Coste K. Functional connectivity and epileptogenicity of nodular heterotopias: A single-pulse stimulation study. Epilepsia. 2022. 63: 961-73
6. Bourdillon P, Rheims S, Catenoix H, Montavont A, Ostrowsky-Coste K, Isnard J. Surgical techniques: Stereoelectroencephalography-guided radiofrequencythermocoagulation (SEEG-guided RF-TC). Seizure. 2020. 77: 64-8
7. Cossu M, Fuschillo D, Cardinale F, Castana L, Francione S, Nobili L. Stereo-EEG-guided radio-frequency thermocoagulations of epileptogenic grey-matter nodular heterotopy. J Neurol Neurosurg Psychiatry. 2014. 85: 611-7
8. Cossu M, Fuschillo D, Casaceli G, Pelliccia V, Castana L, Mai R. Stereoelectroencephalography-guided radiofrequency thermocoagulation in the epileptogenic zone: A retrospective study on 89 cases. J Neurosurg. 2015. 123: 1358-67
9. Cossu M, Mirandola L, Tassi L. RF-ablation in periventricular heterotopia-related epilepsy. Epilepsy Res. 2018. 142: 121-5
10. Curry DJ, Gowda A, McNichols RJ, Wilfong AA. MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav. 2012. 24: 408-14
11. Daniel RT, Meagher-Villemure K, Farmer JP, Andermann F, Villemure JG. Posterior quadrantic epilepsy surgery: Technical variants, surgical anatomy, and case series. Epilepsia. 2007. 48: 1429-37
12. Daniel RT, Meagher-Villemure K, Roulet E, Villemure JG. Surgical treatment of temporoparietooccipital cortical dysplasia in infants: Report of two cases. Epilepsia. 2004. 45: 872-6
13. Deleo F, Hong SJ, Fadaie F, Caldairou B, Krystal S, Bernasconi N. Whole-brain multimodal MRI phenotyping of periventricular nodular heterotopia. Neurology. 2020. 95: e2418-26
14. Dubeau F, Tampieri D, Lee N, Andermann E, Carpenter S, Leblanc R. Periventricular and subcortical nodular heterotopia A study of 33 patients. Brain. 1995. 118: 1273-87
15. Esquenazi Y, Kalamangalam GP, Slater JD, Knowlton RC, Friedman E, Morris SA. Stereotactic laser ablation of epileptogenic periventricular nodular heterotopia. Epilepsy Res. 2014. 108: 547-54
16. Felker MV, Walker LM, Sokol DK, Edwards-Brown M, Chang BS. Early cognitive and behavioral problems in children with nodular heterotopia. Epilepsy Behav. 2011. 22: 523-6
17. Hadzine Y, Elmekkaoui A, Benlenda O, Wakrim S, Nassik H. Periventricular nodular heterotopy of the gray matter: A case report. Radiol Case Rep. 2022. 17: 3291-3
18. Hoppe C, Helmstaedter C. Laser interstitial thermotherapy (LiTT) in pediatric epilepsy surgery. Seizure. 2020. 77: 69-75
19. Ivanova MV, Akinina YS, Soloukhina OA, Iskra EV, Buivolova OV, Chrabaszcz AV. The Russian Aphasia Test: The first comprehensive, quantitative, standardized, and computerized aphasia language battery in Russian. PLoS One. 2021. 16: e0258946
20. Khoo HM, Gotman J, Hall JA, Dubeau F. Treatment of epilepsy associated with periventricular nodular heterotopia. Curr Neurol Neurosci Rep. 2020. 20: 59
21. Krishna V, Sammartino F, Rezai A. A review of the current therapies, challenges, and future directions of transcranial focused ultrasound technology: Advances in diagnosis and treatment. JAMA Neurol. 2018. 75: 246
22. Mirza FA, Hall JA. Radiofrequency thermocoagulation in refractory focal epilepsy: The montreal neurological institute experience. Can J Neurol Sci. 2021. 48: 626-39
23. Parrini E. Periventricular heterotopia: Phenotypic heterogeneity and correlation with Filamin A mutations. Brain. 2006. 129: 1892-906
24. Passarelli V, Moreira CH. Periventricular nodular heterotopia: Pathogenesis, epileptogenesis and implications in higher cerebral functions. Mol Cell Epilepsy. 2014. 1: e20
25. Quigg M, Harden C. Minimally invasive techniques for epilepsy surgery: Stereotactic radiosurgery and other technologies: A review. J Neurosurg. 2014. 121: 232-40
26. Scherer C, Schuele S, Minotti L, Chabardes S, Hoffmann D, Kahane P. Intrinsic epileptogenicity of an isolated periventricular nodular heterotopia. Neurology. 2005. 65: 495-6
27. Stefan H, Nimsky C, Scheler G, Rampp S, Hopfengärtner R, Hammen T. Periventricular nodular heterotopia: A challenge for epilepsy surgery. Seizure. 2007. 16: 81-6
28. Stepanenko AY, Arkhipova NA, Pronin IN, Shishkina LV, Lebedeva AV, Guekht AB. Partial disconnection procedure in a patient with bilateral lesions (case report). Epilepsy Behav Case Rep. 2013. 1: 45-9
29. Thompson SA, Kalamangalam GP, Tandon N. Intracranial evaluation and laser ablation for epilepsy with periventricular nodular heterotopia. Seizure. 2016. 41: 211-6
30. Tinuper P, D’Orsi G, Bisulli F, Zaniboni A, Piraccini A, Bernardi B. Malformation of cortical development in adult patients. Epileptic Disord. 2003. 5: S85-90
31. Wu C, Sperling MR, Falowski SM, Chitale AV, Werner-Wasik M, Evans JJ. Radiosurgery for the treatment of dominant hemisphere periventricular heterotopia and intractable epilepsy in a series of three patients. Epilepsy Behav Case Rep. 2013. 1: 1-6
32. Zimmerman RA, Bilaniuk LT, Grossman RI. Computed tomography in migratory disorders of human brain development. Neuroradiology. 1983. 25: 257-63