- Department of Head and Neck, Unidad de Neurociencias, Instituto Nacional de Cancerología, Mexico City, Mexico,
- Department of Investigación Biomédica, Unidad de Investigación Biomédica en Cáncer, Laboratorio de Genómica, Instituto Nacional de Cancerología, Mexico City, Mexico,
- Department of Neurosurgery, National Autonomous University of Mexico, Durango, Mexico
- Department of Neurosurgery, Servicio of the 1ro de Octubre Hospital of the Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado, Instituto Politécnico Nacional, México City, Mexico,
- Department of Neurosurgery, Peoples’ Friendship University of Russia, Moscow, Russian Federation,
- Department of Neurosurgery, Azienda Ospedaliero Universitaria Pisana, Pisa, Italy.
Correspondence Address:
Nicola Montemurro, Department of Neurosurgery, Azienda Ospedaliero Universitaria Pisana, Pisa, Italy
DOI:10.25259/SNI_1016_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Gervith Reyes-Soto1, Jose F. Carrillo-Hernández2, Bernardo Cacho-Díaz1, Carlos Salvador Ovalle3, Carlos Castillo-Rangel4, Renat Nurmukhametov5, Gennady Chmutin5, Manuel De Jesus Encarnacion Ramirez5, Nicola Montemurro6. Surgical treatment of orbital tumors in a single center: Analysis and results. 05-Apr-2024;15:122
How to cite this URL: Gervith Reyes-Soto1, Jose F. Carrillo-Hernández2, Bernardo Cacho-Díaz1, Carlos Salvador Ovalle3, Carlos Castillo-Rangel4, Renat Nurmukhametov5, Gennady Chmutin5, Manuel De Jesus Encarnacion Ramirez5, Nicola Montemurro6. Surgical treatment of orbital tumors in a single center: Analysis and results. 05-Apr-2024;15:122. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12841
Abstract
Background: Orbital tumors, arising within the bony orbit and its contents, present diverse challenges due to their varied origins and complex anatomical context. These tumors, classified as primary, secondary, or metastatic, are further subdivided into intraconal and extraconal based on their relationship with the muscle cone. This classification significantly influences surgical approach and management. This study highlights surgical experiences with orbital tumors, underscoring the importance of tailored surgical approaches based on the lesion’s site and its proximity to the optic nerve.
Methods: This retrospective study at the National Institute of Cancer’s Head and Neck Department (2005–2014) analyzed 29 patients with orbital tumors treated with surgery, radiotherapy, chemotherapy, or combinations of them. Patient demographics, tumor characteristics, and treatment responses were evaluated using computed tomography (CT), magnetic resonance imaging, and positron emission tomography-CT imaging. Malignant tumors often required orbital exenteration and reconstruction, highlighting the study’s commitment to advancing orbital tumor treatment.
Results: 29 patients (18 females and 11 males, age 18–88 years, mean 53.5 years) with orbital tumors exhibited symptoms such as decreased vision and exophthalmos. Tumors included primary lesions like choroidal melanoma and secondary types like epidermoid carcinoma. Treatments varied, involving a multidisciplinary team for surgical approaches like exenteration, with follow-up from 1 to 9 years. Radiotherapy and chemotherapy were used for specific cases.
Conclusion: Our study underscores the need for a multidisciplinary approach in treating orbital tumors, involving various surgical specialists and advanced technologies like neuronavigation for tailored treatment. The integration of surgery with radiotherapy and chemotherapy highlights the effectiveness of multidimensional treatment strategies.
Keywords: Neuro-oncology, Orbital tumor, Retrospective study, Surgical outcome
INTRODUCTION
The orbital tumors originate from the bony orbit and its content and constitute a diversity of lesions with several forms of management.[
Orbital tumors are divided anatomically into intraconal and extraconal; this classification is according to the relationship between the tumor and muscle cone.[
There are many studies of the orbital tumors about their origins and locations in the orbit,[
Anatomically, orbital tumors are further subdivided into intraconal and extraconal categories. This distinction is based on the tumor’s relationship with the muscle cone, a critical factor in determining the surgical approach and management strategy.[
The challenge in treating orbital tumors lies not only in the removal of the tumor itself but also in preserving the intricate functions and esthetics of the eye and surrounding structures. Therefore, a nuanced understanding of orbital anatomy, coupled with advances in surgical techniques and interdisciplinary collaboration, is essential for successful outcomes in the treatment of orbital tumors.[
MATERIALS AND METHODS
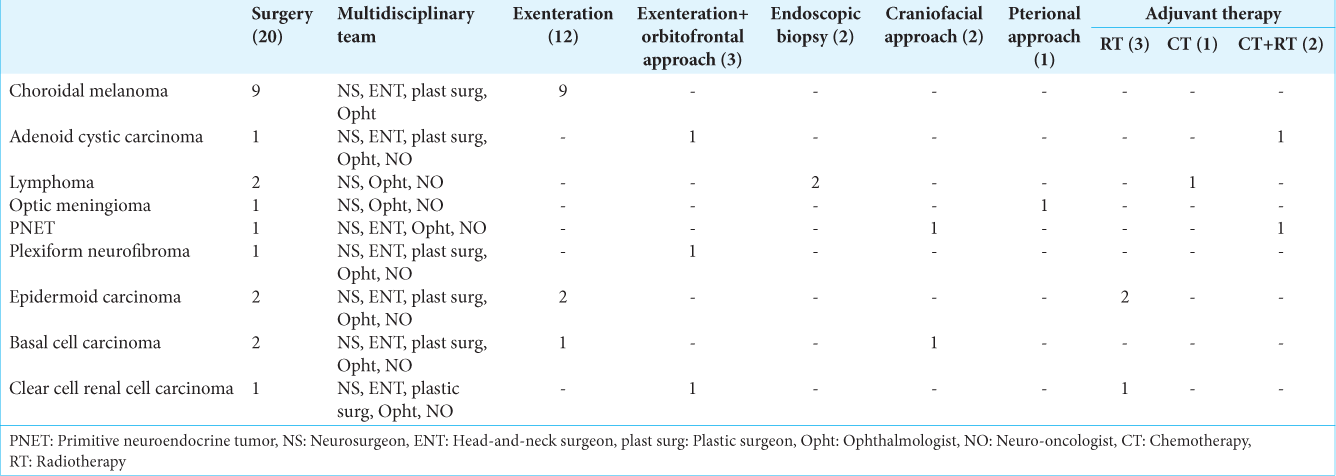
This study presents a retrospective analysis conducted at the Head and Neck Department of the National Institute of Cancer, covering a period from 2005 to 2014. Inclusion criteria were (1) histological confirmation of tumor of the orbit, (2) having available radiological exams before and after treatment, and (3) comprehensive follow-up data. Twenty-nine patients with various types of orbital tumors fulfilled the inclusion criteria and were included in the study. Two patients were excluded from this study as they were lost to follow-up. These individuals underwent diverse treatment modalities, including surgical interventions, radiotherapy, chemotherapy, or a combination thereof. The treatment and subsequent monitoring of these patients were entrusted to a skilled multidisciplinary team comprising neurosurgeons, head-and-neck surgeons, plastic surgeons, ophthalmologists, and neuro-oncologists. This collaborative approach allowed for a holistic assessment and management of each case. Our evaluation process involved an in-depth examination of patient demographics and a thorough analysis of tumor characteristics. Diagnostic methodologies included the utilization of computed tomography (CT) scans, magnetic resonance imaging (MRI), and endoscopic biopsies. Follow-up assessments were conducted using CT, MRI, and positron emission tomography-CT (PET-CT) imaging to monitor disease progression and response to treatment. We categorized the patient population into three distinct groups based on tumor origin: primary lesions, secondary lesions, and metastatic tumors. The surgical management strategies were carefully tailored, considering several pivotal factors. These included the tumor’s location relative to the optic nerve, guiding our choice of surgical approach (craniotomy for superior/lateral positions, endoscopic approach for medial/posterior positions, craniofacial approach, and/or endoscopy for inferior positions); the origin and size of the tumor; and the intended surgical goal, which ranged from biopsy and debulking to total resection.
The orbital tumors were further classified based on their positioning in relation to the muscle cone, categorized as either extraconal or intraconal. The intraconal space, encircled by the conus connecting the rectus muscles, was differentiated from the extraconal area, which lies outside the muscle cone and houses fat and the lacrimal gland. The selection of surgical technique (endoscopic, microscopic, or hybrid) was determined by the tumor’s specific location. Neuronavigation technology played a vital role in all these procedures. The surgical techniques employed included the orbito-fronto-zygomatic approach or the orbitofronto approach as per Zambraski’s methodology, with the endoscopic endonasal technique reserved for biopsy and debulking procedures, particularly when tumors were situated medially and posteriorly in relation to the optic nerve. In instances of malignant tumors, orbital exenteration was performed, followed by adjunctive radiotherapy, chemotherapy, or a combination of both. This extensive procedure entailed the removal of all orbital contents, including the globe, muscles, fat, and lids. Furthermore, when lesions involved the bony structures of the orbit and periorbit, orbital wall reconstruction was indicated. In cases of exenteration, patients were also fitted with ocular prostheses. This comprehensive and methodical approach underscores our commitment to advancing the understanding and treatment of orbital tumors. By integrating cutting-edge diagnostic techniques, nuanced surgical methods, and interdisciplinary collaboration, we strive to enhance patient outcomes in this complex and challenging field.
RESULTS
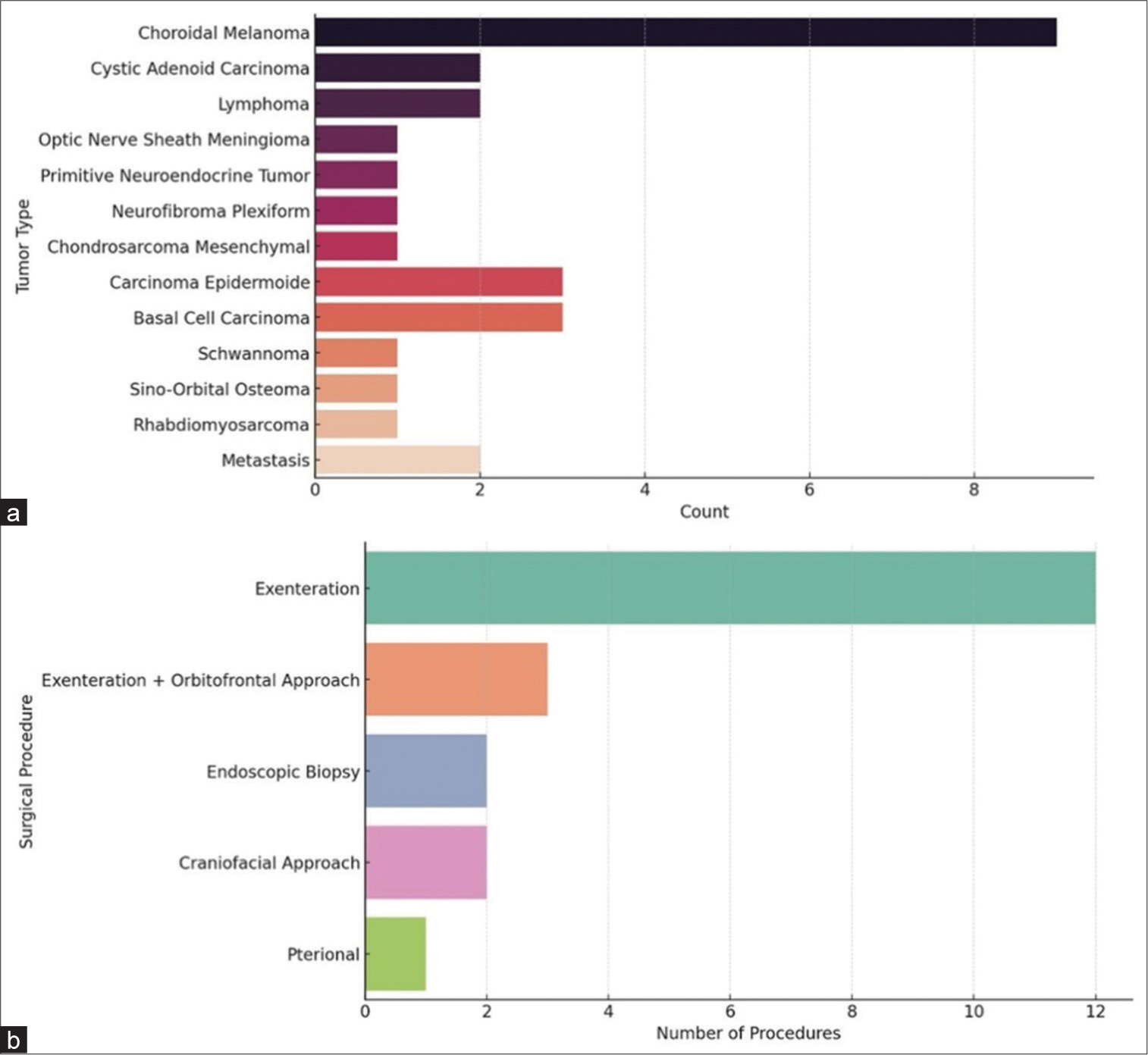
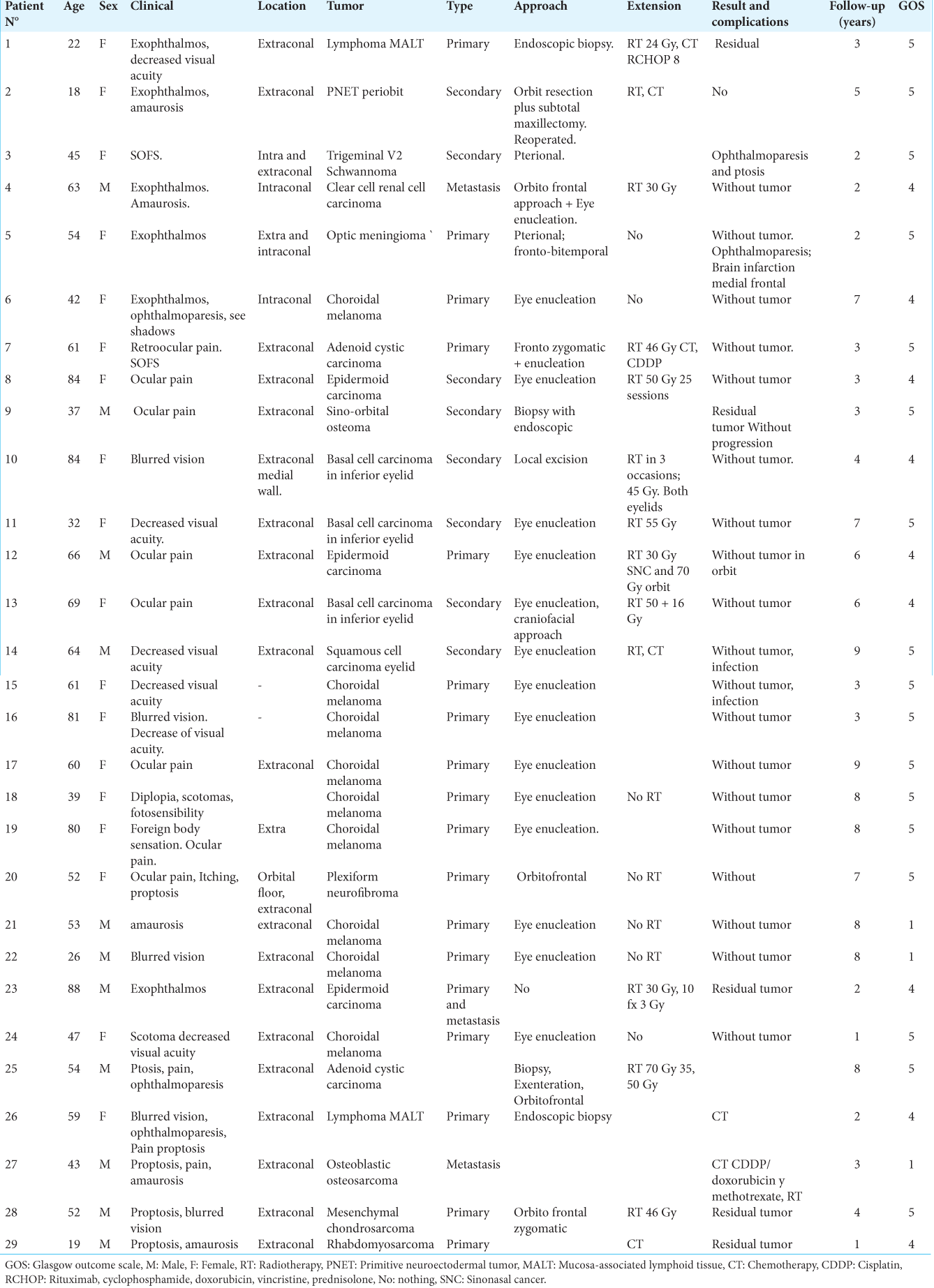
We studied 29 patients (18 females and 11 males) with ages ranging from 18 to 88 years (mean age 53.5 years). The major clinical manifestations were decreased visual acuity (39.28%), exophthalmos (39.28%), local pain (32.4%), ophthalmoparesis (17.8%), and amaurosis (14.28%). Among all patients 17 cases were primary tumors, 10 cases were secondary lesions and 2 cases were metastasis. The primary lesions were choroidal melanoma (9 cases), adenoid cystic carcinoma (2 cases), lymphoma (2 cases), and one single case of optic nerve sheath meningioma, primitive neuroectodermal tumor (PNET), plexiform neurofibroma and one mesenchymal chondrosarcoma; whereas secondary lesions were epidermoid carcinoma (3 cases), basal cell carcinoma (3 cases), one squamous cell carcinoma, one schwannoma, one sino-orbital osteoma, one rhabdomyosarcoma and two metastasis (clear cell renal cell carcinoma and osteoblastic osteosarcoma) [
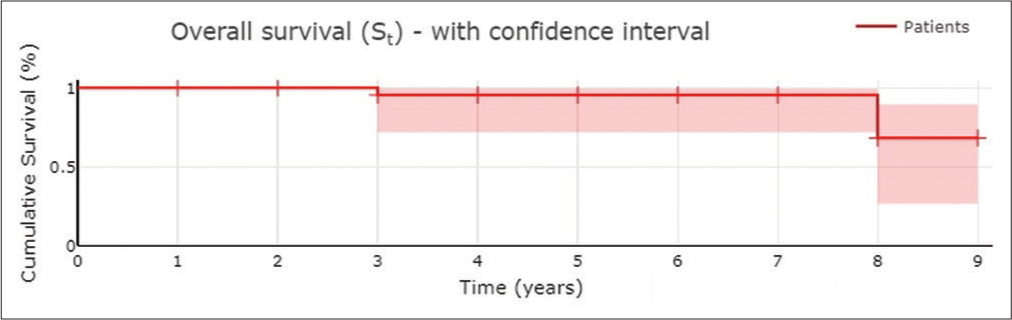
The radiotherapy was performed on three patients with epidermoid carcinoma and one patient with metastasis. Chemotherapy was performed in one case of lymphoma, metastasis (osteosarcoma), and rhabdomyosarcoma. Chemotherapy plus radiotherapy was performed on one patient with adenoid cystic carcinoma and PNET. There were no patients who died in the 30 days following surgery. Complications reported were infection in 2 (6.9%) patients and brain infarction in 1 (3.4%) patient. In most of the patients, complete (82.8%) or subtotal (17.2%) resections were achieved. The follow-up was done from 1 to 9 years. Glasgow Outcome Scale was used, with the following results: 17 (58.6%) patients with GOS grade 5, 9 (31.0%) patients with GOS grade 4, and 3 (10.3%) patients died at the last follow-up.
DISCUSSION
Shinder et al.[
According to the location of the orbital tumor, Darsaut et al.[
Markowski et al.[
The best surgical approach is usually decided on the location of the tumor in the orbit in relationship with the optic nerve, the size of the lesion, the type of the tumor, and the goal of the surgery (biopsy, total resection, and partial resection). Here, we reported the two main surgical approaches to the orbit, which is to say, the external surgical orbital approach and the endoscopic endonasal transorbital approach.
External surgical approaches
A transcranial approach (pterional and orbitofrontal approach) is suggested when the tumor is located at the orbital apex or to the superior orbital fissure, as it provides the best exposure of the orbital cavity.[
The lateral orbital approach was first described by Krönlein,[
Endoscopic endonasal transorbital approach
There are few endoscopic reports on the management of orbital tumors.[
Furthermore, the use of an exoscope in orbital tumor surgeries is relatively new and is part of the ongoing evolution of surgical techniques. The incorporation of exoscopic technology into the management of orbital tumors represents a promising development, representing a step forward towards minimally invasive procedures, with the aim of reducing patient recovery times and improving surgical precision. Exoscope improves surgical visualization, allows for greater precision in tumor excision and, when used in conjunction with endoscopic techniques, provides a comprehensive approach to the management of complex cases. Likewise, the use of augmented reality and telemedicine in the preoperative planning of orbital pathologies has been shown to improve the accuracy and precision of the incision and enable the bioprinting procedure.[
The reconstruction of the orbital
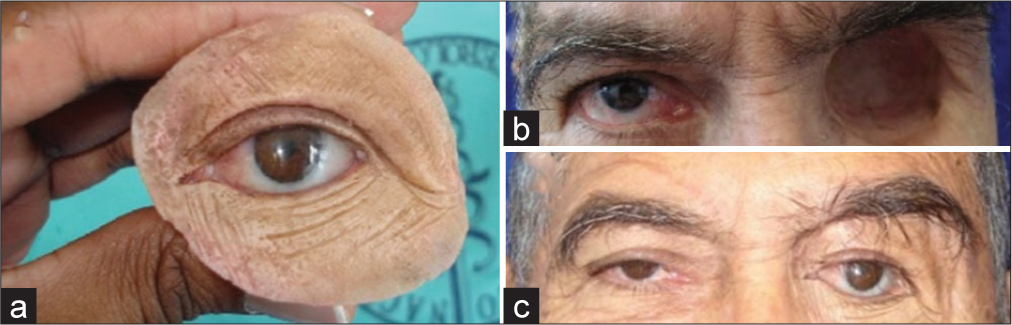
The orbital prosthesis can improve the patient’s appearance, enable early rehabilitation, shorten surgery and hospitalization time, lower treatment costs, and allow early psychosocial reintegration.[
The use of an orbital prosthesis offers several advantages. It significantly enhances the patient’s appearance and facilitates early rehabilitation. This is crucial in reducing the duration of surgery and hospitalization, which in turn lowers treatment costs.[
In cases of orbital exenteration, which involves the removal of all orbital contents including the eye, eyelids and surrounding tissues, an orbital prosthesis becomes extremely important. [
Limitations of the study
This study has some limitations, as it reflects the experience of a single institution. The study is based on a limited number of cases, which may not provide a comprehensive representation of the broader patient population with similar conditions. This relatively small sample size limits the generalizability of the findings and may not capture the full spectrum of potential outcomes and complications associated with the surgical technique. Multicenter studies are needed to validate these findings.
CONCLUSION
Our research emphasizes the importance of a multidisciplinary approach, combining the expertise of neurosurgeons, head-and-neck surgeons, plastic surgeons, ophthalmologists, and neuro-oncologists. This collaborative effort enables tailored treatment strategies based on tumor type, location, and relationship with the optic nerve, thereby optimizing patient outcomes. The study’s findings highlight the necessity of precise surgical techniques and the pivotal role of advanced technologies such as neuronavigation, endoscopic equipment, and exoscopes in enhancing surgical precision and minimizing invasiveness. The detailed analysis of surgical approaches based on tumor location and characteristics underscores the need for individualized treatment plans. Furthermore, the successful integration of surgical interventions with radiotherapy and chemotherapy in certain cases demonstrates the potential for multidimensional treatment plans in managing these complex cases.
Ethical Approval
The author(s) declare that they have taken the ethical approval from IRB of Department of Head and Neck, Unidad de Neurociencias, Instituto Nacional de Cancerología, Mexico City, Mexico (06/2023).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abuzayed B, Tanriover N, Gazioglu N, Eraslan BS, Akar Z. Endoscopic endonasal approach to the orbital apex and medial orbital wall: Anatomic study and clinical applications. J Craniofac Surg. 2009. 20: 1594-600
2. Akiki RK, Jehle CC, Crozier J, Woo AS. Using 3D printing and mirror image modeling in orbital floor reconstruction. J Craniofac Surg. 2021. 32: 2465-7
3. Alvarez Aquino A, Ramirez MJE, Bozkurt I, Asprilla González JA, Goncharov E, Caballero AD. Treatment of intracranial tumors with stereotactic radiosurgery: Short-term results from cuba. Cureus. 2022. 14: e29955
4. Bansal R, Honavar SG, Talloju SS, Mulay K. Orbital dermoid cyst. Indian J Ophthalmol. 2022. 70: 709
5. Berg EJ, Clark JD. Orbital Rhabdomyosarcoma. N Engl J Med. 2023. 388: e4
6. Berke RN. A modified Kronlein operation. AMA Arch Ophthalmol. 1954. 51: 609-32
7. Blumer M, Pejicic R, Gander T, Johner JP, Held U, Wagner ME. Customized titanium reconstruction of orbital fractures using a mirroring technique for virtual reconstruction and 3D model printing. J Oral Maxillofac Surg. 2021. 79: 200.e1-200.e9
8. Boari N, Gagliardi F, Castellazzi P, Mortini P. Surgical treatment of orbital cavernomas: Clinical and functional outcome in a series of 20 patients. Acta Neurochir (Wien). 2011. 153: 491-8
9. Bruce CN, Kroft SH, Harris GJ. Orbital MALT lymphoma with amyloid deposition. Orbit. 2023. 1: 1-5
10. Darsaut TE, Lanzino G, Lopes MB, Newman S. An introductory overview of orbital tumors. Neurosurg Focus. 2001. 10: E1
11. Das S, Montemurro N, Ashfaq M, Ghosh D, Sarker AC, Khan AH. Resolution of papilledema following ventriculoperitoneal shunt or endoscopic third ventriculostomy for obstructive hydrocephalus: A pilot study. Medicina (Kaunas). 2022. 58: 281
12. Douglas VP, Douglas KAA, Cestari DM. Optic nerve sheath meningioma. Curr Opin Ophthalmol. 2020. 31: 455-61
13. Dubois L, Eddy Becking AG. Up-to-date on orbital trauma and reconstruction. Atlas Oral Maxillofac Surg Clin North Am. 2021. 29: xi-xii
14. Dubron K, Verbist M, Jacobs R, Olszewski R, Shaheen E, Willaert R. Augmented and virtual reality for preoperative trauma planning, focusing on orbital reconstructions: A systematic review. J Clin Med. 2023. 12: 5203
15. Encarnacion-Ramirez MJ, Aquino AA, Castillo REB, Melo-Guzmán G, López-Vujnovic D, Blas A. Surgical management of a penetrating drill bit injury to the skull base. Surg Neurol Int. 2022. 13: 49
16. Fang Y, Peng Z, Wang Y, Gao K, Liu Y, Fan R. Current opinions on diagnosis and treatment of adenoid cystic carcinoma. Oral Oncol. 2022. 130: 105945
17. Fortunato GM, Sigismondi S, Nicoletta M, Condino S, Montemurro N, Vozzi G. Analysis of the robotic-based In situ bioprinting workflow for the regeneration of damaged tissues through a case study. Bioengineering (Basel). 2023. 10: 560
18. Ganz JC. Orbital indications. Prog Brain Res. 2022. 268: 315-27
19. Goh EZ, Bullis S, Beech N, Johnson NR. Intraoperative computed tomography for orbital reconstruction: A systematic review. Int J Oral Maxillofac Surg. 2024. 53: 127-32
20. Gupta S, Mehrotra D, Singh PK, Vignesh UU V, Bhave S, Katrolia R. Quality of life after reconstruction of traumatic orbital floor defects using titanium mesh and medpore: A randomised controlled trial. J Oral Biol Craniofac Res. 2021. 11: 200-3
21. Hajibandeh J, Lee C. Patient-specific implants in orbital reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2022. 30: 230-5
22. Hassan WM, Alfaar AS, Bakry MS, Ezzat S. Orbital tumors in USA: Difference in survival patterns. Cancer Epidemiol. 2014. 38: 515-22
23. Hatamleh MM, Watson J, Srinivasan D. Closed-eye orbital prosthesis: A clinical report. J Prosthet Dent. 2015. 113: 246-9
24. Huang A, Su M, Jing Y, He S, He X, Ma J. Orbital primary solitary fibrous tumor: A proposed recurrence risk prediction model based on 92 cases. Hum Pathol. 2023. 137: 85-93
25. Hudecki A, Wolany W, Likus W, Markowski J, Wilk R, KolanoBurian A. Orbital reconstruction-applied materials, therapeutic agents and clinical problems of restoration of defects. Eur J Pharmacol. 2021. 892: 173766
26. Koutourousiou M, Gardner PA, Stefko ST, Paluzzi A, Fernandez-Miranda JC, Snyderman CH. Combined endoscopic endonasal transorbital approach with transconjunctival-medial orbitotomy for excisional biopsy of the optic nerve: Technical note. J Neurol Surg Rep. 2012. 73: 52-6
27. Kronlein R. Zur Pathologie und Behandlung der Dermoidcysten der Orbita [On the pathology and treatment of dermoid cysts of the orbit]. Beitr Klin Chir. 1888. 4: 149
28. Li R, Ren M, Liu L. Infrequent bilateral orbital tumors. Ophthalmology. 2023. 130: 747
29. Margalit N, Ezer H, Fliss DM, Naftaliev E, Nossek E, Kesler A. Orbital tumors treated using transcranial approaches: Surgical technique and neuroophthalmogical results in 41 patients. Neurosurg Focus. 2007. 23: E11
30. Markowski J, Jagosz-Kandziora E, Likus W, Pająk J, MrukwaKominek E, Paluch J. Primary orbital tumors: A review of 122 cases during a 23-year period: A histo-clinical study in material from the ENT department of the medical university of Silesia. Med Sci Monit. 2014. 20: 988-94
31. Martins C, Costa E Silva IE, Campero A, Yasuda A, Aguiar LR, Tatagiba M. Microsurgical anatomy of the orbit: The rule of seven. Anat Res Int. 2011. 2011: 468727
32. Mohankumar A, Gurnani B, editors. Orbital apex syndrome. StatPearls. Treasure Island, FL: StatPearls Publishing; 2023. 1: 1
33. Montemurro N, Condino S, Carbone M, Cattari N, D’Amato R, Cutolo F. Brain tumor and augmented reality: New technologies for the future. Int J Environ Res Public Health. 2022. 19: 6347
34. Montemurro N, Scerrati A, Ricciardi L, Trevisi G. The exoscope in neurosurgery: An overview of the current literature of intraoperative use in brain and spine surgery. J Clin Med. 2021. 11:
35. Montemurro N. Telemedicine: Could it represent a new problem for spine surgeons to solve?. Global Spine J. 2022. 12: 1306-7
36. Müller-Forell W, Pitz S. Orbital pathology. Eur J Radiol. 2004. 49: 105-42
37. Murdock N, Mahan M, Chou E, editors. Benign orbital tumors. StatPearls. Treasure Island, FL: StatPearls Publishing; 2023. 8: 1
38. Ohtsuka K, Hashimoto M, Suzuki Y. A review of 244 orbital tumors in Japanese patients during a 21-year period: Origins and locations. Jpn J Ophthalmol. 2005. 49: 49-55
39. Olsen TG, Heegaard S. Orbital lymphoma. Surv Ophthalmol. 2019. 64: 45-66
40. Pahwa B, Singh N, Singh G, Chavda V, Montemurro N, Chaurasia B. Surgical approaches to cavernous sinus: A narrative review of the literature with anatomical drawings. J Neurol Surg A Cent Eur Neurosurg. 2022. 4: 250-60
41. Paluzzi A, Gardner PA, Fernandez-Miranda JC, Tormenti MJ, Stefko ST, Snyderman CH. “Round-the-Clock” surgical access to the orbit. J Neurol Surg B Skull Base. 2015. 76: 12-24
42. Park HJ, Yang SH, Kim IS, Sung JH, Son BC, Lee SW. Surgical treatment of orbital tumors at a single institution. J Korean Neurosurg Soc. 2008. 44: 146-50
43. Peron S, Paulli S, Stefini R. Case report: High-definition 4K-3D exoscope for removal of an orbital cavernous hemangioma using a transpalpebral approach. Front Surg. 2021. 8: 671423
44. Ponto KA, Brockmann MA, Koutsimpelas D, Heider J, Ringel FA, Heindl LM. Exzisionale chirurgie orbitaler tumoren [Excisional surgery of orbital tumors]. Ophthalmologe. 2021. 118: 995-1003
45. Potter JK, Malmquist M, Ellis E. Biomaterials for reconstruction of the internal orbit. Oral Maxillofac Surg Clin North Am. 2012. 24: 609-27
46. Ramirez ME, Peralta I, Nurmukhametov R, Castillo REB, Castro JS, Volovich A. Expanding access to microneurosurgery in low-resource settings: Feasibility of a low-cost exoscope in transforaminal lumbar interbody fusion. J Neurosci Rural Pract. 2023. 14: 156-60
47. Rathee M, Chahal S, Alam M, Jain P, Divakar S, Singh S. Prosthetic rehabilitation following segmental maxillectomy confluent with an orbital defect using a hollow orbital prosthesis retained magnetically with an obturator: A case report. J West Afr Coll Surg. 2023. 13: 98-102
48. Ruggiero F, Cercenelli L, Emiliani N, Badiali G, Bevini M, Zucchelli M. Preclinical application of augmented reality in pediatric craniofacial surgery: An accuracy study. J Clin Med. 2023. 12: 2693
49. Seen S, Young S, Lang SS, Lim TC, Amrith S, Sundar G. Orbital implants in orbital fracture reconstruction: A ten-year series. Craniomaxillofac Trauma Reconstr. 2021. 14: 56-63
50. Shapey J, Sabin HI, Danesh-Meyer HV, Kaye AH. Diagnosis and management of optic nerve sheath meningiomas. J Clin Neurosci. 2013. 20: 1045-56
51. Shinder R, Al-Zubidi N, Esmaeli B. Survey of orbital tumors at a comprehensive cancer center in the United States. Head Neck. 2011. 33: 610-4
52. Smith TJ. Novel aspects of orbital fibroblast pathology. J Endocrinol Invest. 2004. 27: 246-53
53. Uhl JF, Sufianov A, Ruiz C, Iakimov Y, Mogorron HJ, Encarnacion Ramirez M. The use of 3D printed models for surgical simulation of cranioplasty in craniosynostosis as training and education. Brain Sci. 2023. 13: 894
54. Weizman N, Horowitz G, Gil Z, Fliss DM. Surgical management of tumors involving the orbit. JAMA Otolaryngol Head Neck Surg. 2013. 139: 841-6
55. Yesensky J, Lebo N. Reconstructive options following orbital exenteration. Curr Opin Otolaryngol Head Neck Surg. 2020. 28: 352-4