- Department of Neurosurgery, National Institute of Neurology and Neurosurgery, Mexico City, Mexico
- Department of Neurosurgery, Salvador Zubirán National Institute of Health Sciences and Nutrition, Mexico City, Mexico.
Correspondence Address:
Rodolfo Villalobos-Diaz, Department of Neurosurgery, National Institute of Neurology and Neurosurgery, Mexico City, Mexico.
DOI:10.25259/SNI_22_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rodolfo Villalobos-Diaz1, Ricardo Marian-Magaña1, Marcos Vinicius Sangrador-Deitos1, Rafael Vazquez-Gregorio1, Luis Alberto Rodriguez-Hernandez1, German Lopez-Valencia1, Jorge Fernando Aragon-Arreola1, Jorge Armando Lara-Olivas1, Gerardo Yoshiaki Guinto-Nishimura1, Aldo Gabriel Eguiluz-Melendez2, Juan Luis Gomez-Amador1. Surgical treatment of pituitary neuroendocrine tumors with coexisting intracranial lesions: A case series and review of the literature. 22-Mar-2024;15:96
How to cite this URL: Rodolfo Villalobos-Diaz1, Ricardo Marian-Magaña1, Marcos Vinicius Sangrador-Deitos1, Rafael Vazquez-Gregorio1, Luis Alberto Rodriguez-Hernandez1, German Lopez-Valencia1, Jorge Fernando Aragon-Arreola1, Jorge Armando Lara-Olivas1, Gerardo Yoshiaki Guinto-Nishimura1, Aldo Gabriel Eguiluz-Melendez2, Juan Luis Gomez-Amador1. Surgical treatment of pituitary neuroendocrine tumors with coexisting intracranial lesions: A case series and review of the literature. 22-Mar-2024;15:96. Available from: https://surgicalneurologyint.com/surgicalint-articles/12818/
Abstract
Background: Pituitary neuroendocrine tumors (PitNETs) are a diverse group of benign neoplasms that account for a significant proportion of intracranial tumors (13%). The coexistence of PitNET with other intracranial lesions, such as meningiomas and intracranial aneurysms, has been constantly reported in the literature; yet, the pathophysiological mechanisms remain unknown, and the appropriate management is controversial. This study aims to describe the clinical characteristics, surgical treatment, and outcomes of patients with PitNET with coexisting intracranial lesions in a single healthcare center.
Methods: A retrospective analysis was conducted on 12 patients who underwent surgical treatment for PitNET and another intracranial lesion at our single tertiary referral center over 15 years from January 2008 to May 2023.
Results: Among these coexisting lesions, aneurysms were the most commonly found (41.67%), followed by meningiomas (33.33%). Surgical intervention for both lesions was performed in a single-stage procedure for most cases (75%), employing transcranial, endoscopic endonasal, and combined approaches. We found low preoperative Karnofsky Performance Scale scores in three patients, with significant differences in functional outcomes.
Conclusion: These findings contribute to the limited knowledge about PitNET coexisting with other intracranial lesions and emphasize the importance of patient-tailored, multidisciplinary management in these unusual scenarios.
Keywords: Aneurysm, Coexisting, Concomitant, Meningioma, Pituitary adenoma, Pituitary neuroendocrine tumor
INTRODUCTION
Pituitary neuroendocrine tumors (PitNETs), previously named pituitary adenomas, represent a heterogeneous group of extra-axial benign tumors, which account for approximately 13% of all intracranial tumors,[
Meningiomas are usually slow-growing tumors derived from the arachnoid cap cells, with an overall incidence of 8.3/100,000, and constitute the most common intracranial benign tumors.[
Different MESH terms have been employed to define the coexistence of more than one lesion in the intracranial space, such as “synchronous,” “concomitant,” “coexisting,” “coincidental,” and “collision tumor.” A distinction should be made in the case of collision tumors, which are defined as two or more histologically different neoplasms coexisting in the same area without histological admixture and, when involving the sellar region, common associations comprise PitNET along with Rathke cleft cysts (most frequent), craniopharyngiomas, metastatic brain tumors, gangliocytomas, and meningiomas, among others.[
Even though the association between PitNET and other intracranial lesions has been well documented, the underlying pathophysiological mechanism remains unclear. Furthermore, there is no actual consensus about the best therapeutic option for such concomitant lesions; however, surgical treatment represents the best therapeutic option in those cases requiring intervention.[
MATERIALS AND METHODS
Patient population
We conducted a retrospective review of the medical records of all patients who were diagnosed with PitNET in coexistence with other intracranial lesions and who were surgically treated in our institution over 15 years (between 2008 and 2023). The review included demographic data, preand post-operative images, operative notes, histopathologic reports, and follow-up medical notes. Patients with a history of cranial radiotherapy and incomplete data in the medical records were excluded from the study. Institutional Review Board approval and informed consent were not required for the present study due to its retrospectiv design, without identifiable personal data.
PitNET were classified as non-functioning (NF) or functioning tumors. The hormonal type of the latter was further defined based on both hormonal profile results and clinical manifestations. The coexisting intracranial lesions were defined based on their histopathologic characteristics obtained from surgical samples; however, in those cases where the acquisition of tissue was not feasible (such as aneurysmal lesions), they were defined based on the preoperative images and intraoperative findings.
Surgical procedures
All surgical procedures were authorized through written informed consent and performed by experienced skull base neurosurgeons. Surgical interventions were classified according to the number of procedures (one-stage vs. two-stage surgery) and the surgical approach(es) performed (TC, endonasal endoscopic, or both), which were further specified.
Statistical analysis
Data are presented as means and standard deviations for continuous variables and as percentages for categorical variables. The Shapiro–Wilk test was used to determine distribution normality. Differences between categorical variables were determined with a Chi-squared test, whereas, for mean comparison of continuous variables, T and Wilcoxon tests were used, according to data distribution. Data were analyzed using STATA 17.
RESULTS
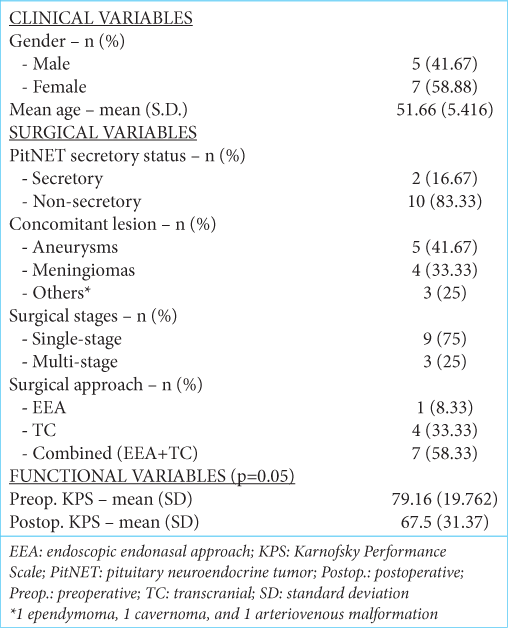
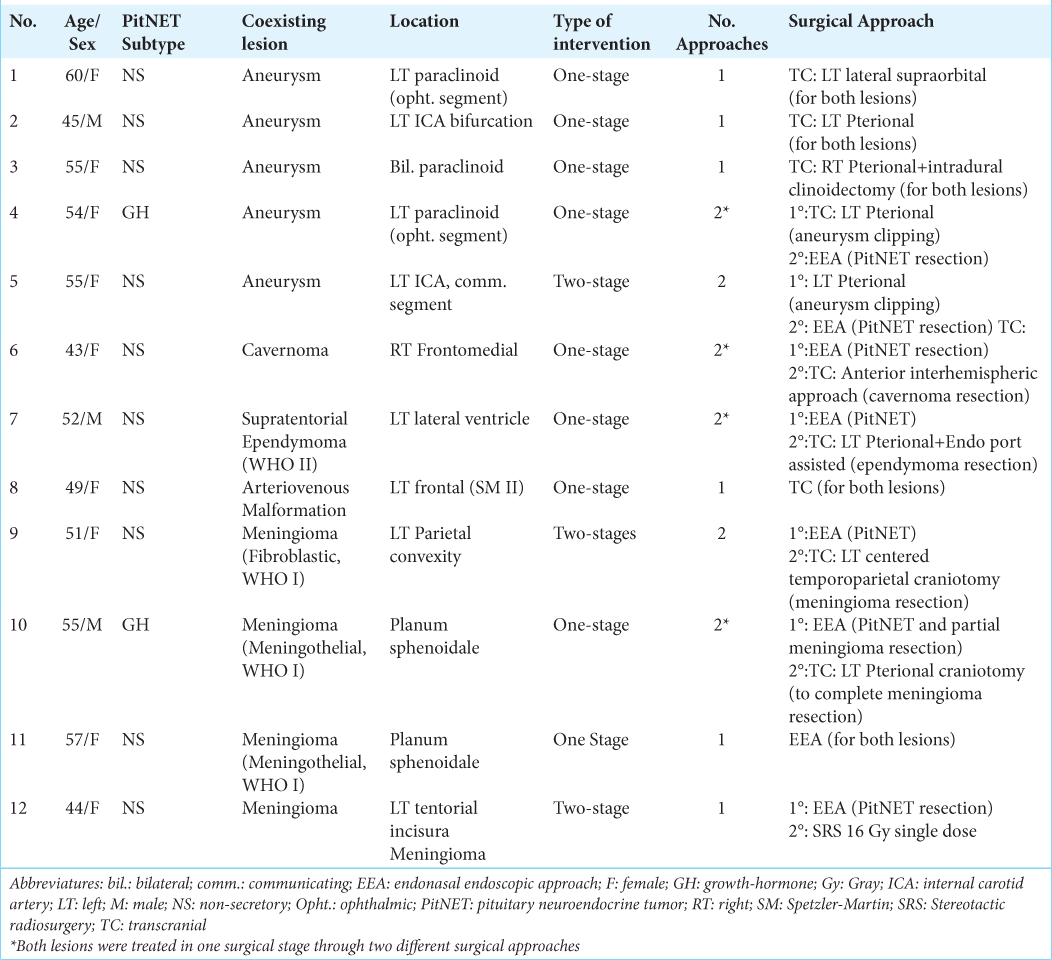
A total of 1666 patients with the diagnosis of PitNET were operated on from January 2008 to May 2023 in our institution. Twelve patients with a coexisting intracranial lesion (0.72%) were included in our study. The mean (standard deviation [SD]) age of patients was 51.66 (5.416) (range 43–60) years, and 5 (41.67%) were men. Regarding PitNET, 10 (83.33%) were NF, and the remaining 2 (16.67%) were documented as growth hormone (GH)-secreting lesions. Aneurysms were the most common concomitant lesions, representing 5 (41.67%) of the total, followed by 4 (33.33%) meningiomas, 1 (8.33%) ependymoma, one cavernoma, and one arteriovenous malformation. As for the surgical approach chosen, 9 (75%) patients were intervened for both lesions in a single-stage procedure being the TC route performed in 4 (33.33%) patients; meanwhile, 1 (8.33%) patient was intervened through an endoscopic endonasal approach (EEA), and 7 (58.33%) with a combined TC/EEA combined approach. Three patients presented with low preoperative Karnofsky Performance Scale scores (70 or less) with a mean (SD) value of 79.16 (19.762) (range 30-90). The mean (SD) postoperative KPS was 67.5 (31.37). Significant differences were found among functional outcomes (P = 0.05) [
Representative cases
Case 1
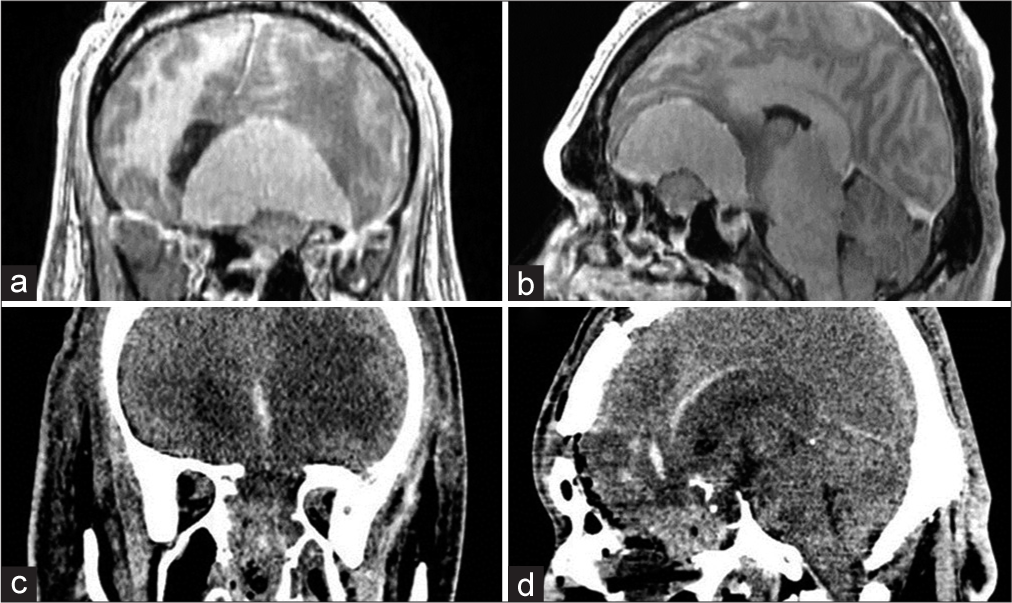
A 54-year-old man with a 15-year history of acromegalic physical changes was referred to our emergency department due to the gradual onset of mental status and behavioral changes. On physical examination, the patient had evident acromegalic features; disorientation and inattention stood out on neurological examination. Fundoscopic examination revealed bilateral papillary atrophy. Eye movements were unrestricted, and his right pupil was mydriatic and with diminished response to light. The rest of the exploration was not assessable. Brain magnetic resonance imaging (MRI) with contrast revealed a large, homogeneously enhancing anterior skull base lesion originating from and conditioning hyperostosis of the planum sphenoidale, compatible with a meningioma. In addition, a homogeneously enhancing sellar lesion with some areas of T1-weighted imaging shortening, compatible with a PitNET, was observed [
Figure 1:
(a and b) Coronal and sagittal T1-post contrast magnetic resonance imaging demonstrated a homogeneously enhancing anterior fossa lesion, dependent on the planum sphenoidale, which conditioned significant hyperostosis of the sphenoid bone. The lesion extended dorsally, leading to a right subfalcine herniation and compression of the frontal horns of the lateral ventricles and corpus callosum. In the sagittal projection, a hyperintense sellar mass confined to the sella turcica was also noted. (c and d) Coronal and sagittal postoperative non-contrast computed tomography revealed total tumoral removal of both meningioma and PitNET, associated with a significant anterior skull base bony defect, which was repaired as mentioned above.
We decided to approach both lesions through a combined EEA and TC approach in one surgical stage. Initially, an EEA trans-tuberculum/trans-planum was performed for both resection of the sellar lesion and vascular control of the meningioma by coagulation of the posterior ethmoidal arteries. Once the endoscopic stage was concluded, a transbasal approach was performed, achieving a Simpson I resection. The anterior skull base was reconstructed with fat graft and pedicled pericranium, and the sellar floor was reconstructed in a multilayered fashion employing inlay fascia lata, fat graft, onlay fascia lata, and a previously harvested nasoseptal flap. Transient diabetes insipidus and hypernatremia arose postoperatively, which were managed satisfactorily, and the patient was discharged after two weeks. At follow-up, biochemical remission was demonstrated; however, visual acuity remained severely impaired.
Case 2
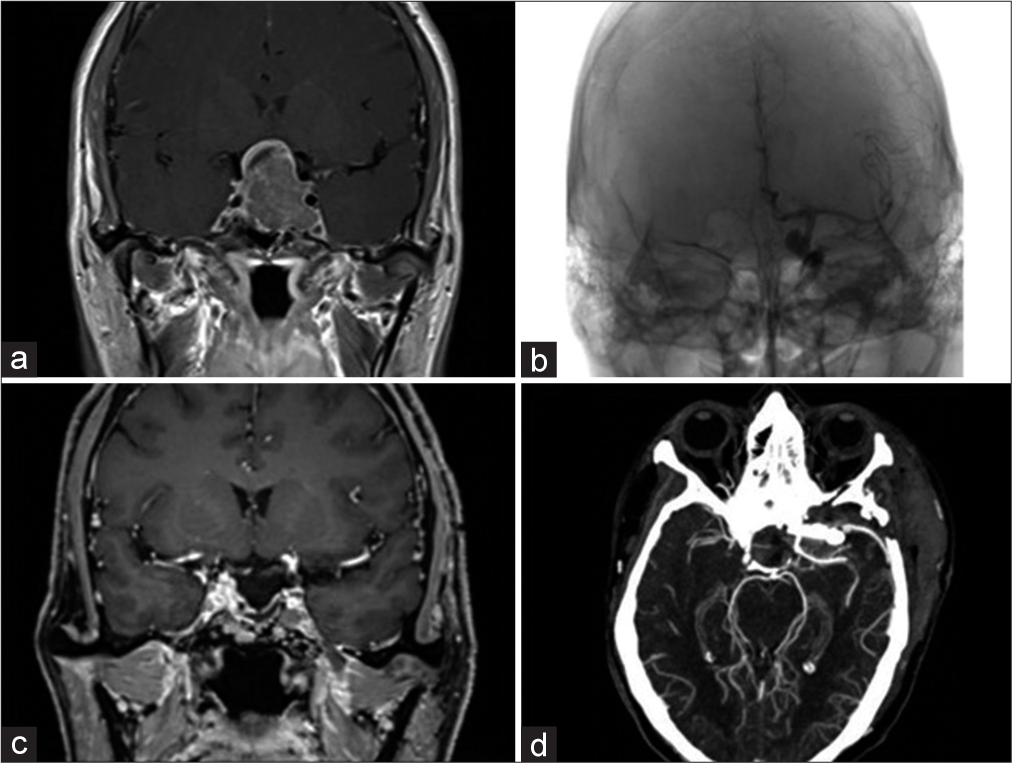
A 55-year-old female with no relevant medical history presented to our department with a 6-year history of headache, joint pain in both hands, as well as acral and facial enlargement. Decreased visual acuity (20/60 bilaterally) and bitemporal hemianopsia were found on ophthalmologic evaluation. Endocrinological testing was relevant for a GH level of 24 ng/mL and IGF-1 of 770 ng/mL (index 3.83). Brain MRI revealed an isointense T1- and T2-weighted imaging sellar lesion, with heterogeneous contrast enhancement, extending upward into the third ventricle and with para sellar extension into the right cavernous sinus [
Figure 2:
(a) Coronal T1-post contrast magnetic resonance imaging (MRI) demonstrated a heterogeneously enhancing sellar lesion, with suprasellar extension into the third ventricle and parasellar extension into the left cavernous sinus. (b) An anteroposterior angiogram of the left internal carotid artery showed a left paraclinoid aneurysm with a dorsomedial projection. (c) Coronal T1-weighted post-contrast MRI obtained two months after surgery revealed a gross total resection of the pituitary neuroendocrine tumors with a small remnant at the left cavernous sinus. (d) Postoperative computed tomography angiography demonstrated total aneurysm occlusion with preservation of the parental vessels and branches
Due to the presence of an aneurysm in the para sellar region and the potential risk of rupture during the EEA resection of the GH-secreting PitNET, we decided to initially perform a microsurgical clipping of the aneurysm through a pterional approach and, subsequently, perform an EEA in a single surgical stage. The treatment of both lesions transcranially was also considered, but due to the enhanced visualization of the sellar region provided by the endoscope and the better tumoral and hormonal control associated, an EEA was favored. During the microsurgical stage, a left extradural anterior clinoidectomy and unroofing of the optic canal were performed to gain access to the distal dural ring and carotid cave for proximal control. Afterward, a left paraclinoid Barami IA, with a dorsomedial projection, was visualized. After thorough dissection, the aneurysm was finally excluded with two clips. Intraoperative fluorescence angiography confirmed the complete exclusion of the aneurysm as well as the hemodynamic integrity of the related vessels. Subsequently, the sellar lesion was resected through an EEA. After opening the sellar dura, a soft tumor was visualized, and gross total resection was performed. A similar reconstruction as the one described in Case 1 was performed. The postoperative course was uneventful. Biochemical remission was achieved, and her vision gradually improved.
DISCUSSION
The coexistence of PitNET with other intracranial lesions is unusual, especially when patients with previous irradiation therapy for pituitary tumors have been excluded from the study. However, in the current medical literature, there are several case reports of PitNET associated with other intracranial lesions, such as craniopharyngiomas,[
The association of IA with PitNET has been constantly reported [
The pattern of distribution of the IA has also been a matter of debate, and a predilection for the anterior circulation has been advised.[
Another controversial association is the hormonal secretion of PitNET and its role in the development of IA. The secretion of GH by the tumor has been proposed as a risk factor for the development of IA.[
The diagnosis of IA associated with PitNET is usually an incidental finding while carrying out preoperative image studies, and most of the IA are silent and unruptured; therefore, the clinical manifestations of the patients are usually due to the mass effect or hormonal secretion of the pituitary tumor.[
Different therapeutic options and protocols have been proposed for the management of PitNET coexisting with IA. Based on the current literature and our institutional experience, it is advisable to treat the IA at the first stage (whether with microsurgical or endovascular procedures) and then proceed with the tumoral resection.[
On the other hand, meningiomas comprised one-third (33.3%) of the intracranial lesions associated with PitNET in the present series, representing the second most frequent lesion. This association in patients with a previous history of intracranial radiation therapy is well known [
The presence of a PitNET coexisting with a distally located meningioma may have several therapeutic options, and they are usually treated independently and according to the presence of symptoms, hormonal secretion, response to medical treatment (prolactinomas), as well as the treatment goals, being the expectant management sometimes the best option.[
CONCLUSION
Although unusual in occurrence, the coexistence of PitNET with other intracranial lesions has been described, representing both IA and meningiomas, the most frequent ones. Despite the low incidence and absence of a clear causative factor for these concomitant lesions, the possibility of this association must be taken into account as part of the preoperative differential diagnosis, and the surgical approach must be tailored to the patient’s symptoms, anatomical location of both lesions, and the suitability for treating both lesion in one surgical stage. Likewise, the present case series provides valuable information to the little knowledge about these unusual concomitant lesions.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abs R, Parizel PM, Willems PJ, Van de Kelft E, Verlooy J, Mahler C. The association of meningioma and pituitary adenoma: Report of seven cases and review of the literature. Eur Neurol. 1993. 33: 416-22
2. Acqui M, Ferrante L, Fraioli B, Cosentino F, Fortuna A, Mastronardi L. Association between intracranial aneurysms and pituitary adenomas. Etiopathogenic hypotheses. Neurochirurgia (Stuttg). 1987. 30: 177-81
3. Acqui M, Ferrante L, Matronardi L, d’Addetta R. Alteration of the collagen type III/type I ratio and intracranial saccular aneurysms in GH-secreting hypophyseal adenomas. Ital J Neurol Sci. 1988. 9: 365-8
4. Alshalawi A, Alkhaibary A, Basalamah A, Alassiri AH, Alarifi A. Glioblastoma and prolactinoma: A rare simultaneous occurrence. J Surg Case Rep. 2019. 2019: rjz030
5. Amirjamshidi A, Mortazavi SA, Shirani M, Saeedinia S, Hanif H. Coexisting pituitary adenoma and suprasellar meningioma-a coincidence or causation effect: report of two cases and review of the literature. J Surg Case Rep. 2017. 2017: rjx039
6. Bao YY, Wu X, Ding H, Hong T. Endoscopic endonasal resection of coexisting pituitary adenoma and meningioma: Two cases report and literature review. Neurochirurgie. 2021. 67: 611-7
7. Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Future Oncol. 2018. 14: 2161-77
8. Cannavò S, Curtò L, Fazio R, Paterniti S, Blandino A, Marafioti T. Coexistence of growth hormone-secreting pituitary adenoma and intracranial meningioma: A case report and review of the literature. J Endocrinol Invest. 1993. 16: 703-8
9. Chernov IV, Kutin MA, Kheyreddin AS, Konovalov AN, Shekhtman OD, Eliava SS. Sochetanie adenom gipofiza i intrakranial’nykh anevrizm [Combination of pituitary adenomas and intracranial aneurysms]. Zh Vopr Neirokhir Im N N Burdenko. 2021. 85: 94-103
10. Daly AF, Beckers A. The epidemiology of pituitary adenomas. Endocrinol Metab Clin North Am. 2020. 49: 347-55
11. De Vries F, Lobatto DJ, Zamanipoor Najafabadi AH, Kleijwegt MC, Verstegen MJ, Schutte PJ. Unexpected concomitant pituitary adenoma and suprasellar meningioma: A case report and review of the literature. Br J Neurosurg. 2023. 37: 677-81
12. Friend KE, Radinsky R, McCutcheon IE. Growth hormone receptor expression and function in meningiomas: Effect of a specific receptor antagonist. J Neurosurg. 1999. 91: 93-9
13. Furtado SV, Venkatesh PK, Ghosal N, Hegde AS. Coexisting intracranial tumors with pituitary adenomas: Genetic association or coincidence?. J Cancer Res Ther. 2010. 6: 221-3
14. Gu Y, Zhong X, Gao Y, He L. Endoscopic endonasal approach for simultaneously treating a pituitary adenoma coexisting with a paraclinoid aneurysm: Illustrative case. J Neurosurg Case Lessons. 2022. 3: CASE22130
15. Hasegawa H, Jentoft ME, Young WF, Lakomkin N, Van Gompel JJ, Link MJ. Collision of craniopharyngioma and pituitary adenoma: Comprehensive review of an extremely rare sellar condition. World Neurosurg. 2021. 149: e51-62
16. Housepian EM, Pool JL. A systematic analysis of intracranial aneurysms from the autopsy file of the Presbyterian Hospital, 1914 to 1956. J Neuropathol Exp Neurol. 1958. 17: 409-23
17. Hu J, Lin Z, Zhang Y, Zheng X, Ran Q, Zhang D. Prevalence of unruptured intracranial aneurysms coexisting with pituitary adenomas. World Neurosurg. 2019. 126: e526-33
18. Imamura J, Okuzono T, Okuzono Y. Fatal epistaxis caused by rupture of an intratumoral aneurysm enclosed by a large prolactinoma: Case report. Neurol Med Chir. 1998. 38: 654-6
19. Jakubowski J, Kendall B. Coincidental aneurysms with tumours of pituitary origin. J Neurol Neurosurg Psychiatry. 1978. 41: 972-9
20. Kim JE, Lim DJ, Hong CK, Joo SP, Yoon SM, Kim BT. Treatment of unruptured intracranial aneurysms in South Korea in 2006: A nationwide multicenter survey from the Korean society of cerebrovascular surgery. J Korean Neurosurg Soc. 2010. 47: 112-8
21. Locatelli M, Spagnoli D, Caroli M, Isalberti M, Branca V, Gaini SM. A potential catastrophic trap: An unusually presenting sellar lesion. Eur J Neurol. 2008. 15: 98-101
22. Mahvash M, Igressa A, Pechlivanis I, Weber F, Charalampaki P. Endoscopic endonasal transsphenoidal approach for resection of a coexistent pituitary macroadenoma and a tuberculum sellae meningioma. Asian J Neurosurg. 2014. 9: 236
23. Meyrignac C, N’Guyen JP, Blatrix C, Degos JD. Méningiome post radique. Complication tardive de l’irradiation de la selle turcique [Post-radiation meningioma, A late complication of the irradiation of the sella turcica]. Nouv Presse Med. 1981. 10: 3246-7
24. Mondragón-Soto MG, Villanueva-Castro E, Tovar-Romero LA, Aragón-Arreola JF, Sangrador-Deitos MV, Cano-Velázquez G. Magnetic resonance imaging finding of coexistence of bilateral paraclinoid aneurysms in a patient with a nonfunctioning macroadenoma, simultaneous resection, and clipping: Illustrative case. J Neurosurg Case Lessons. 2022. 3: CASE21720
25. Nene A, Hong CS, McGuone D, Matouk CC, Omay SB. Staged endovascular treatment of a coexisting parasellar aneurysm and endoscopic resection of a pituitary macroadenoma: Illustrative case. J Neurosurg Case Lessons. 2022. 3: CASE21699
26. Ogawa Y, Watanabe M, Tominaga T. Pituitary adenomas associated with intracranial aneurysms: The clinical characteristics, therapeutic strategies, and possible effects of vascular remodeling factors. J Neurol Surg A Cent Eur Neurosurg. 2022. 83: 555-60
27. Oh MC, Kim EH, Kim SH. Coexistence of intracranial aneurysm in 800 patients with surgically confirmed pituitary adenoma. J Neurosurg. 2012. 116: 942-7
28. Pant B, Arita K, Kurisu K, Tominaga A, Eguchi K, Uozumi T. Incidence of intracranial aneurysm associated with pituitary adenoma. Neurosurg Rev. 1997. 20: 13-7
29. Partington MD, Davis DH. Radiation-induced meningioma after treatment for pituitary adenoma: Case report and literature review. Neurosurgery. 1990. 26: 329-31
30. Prevedello DM, Thomas A, Gardner P, Snyderman CH, Carrau RL, Kassam AB. Endoscopic endonasal resection of a synchronous pituitary adenoma and a tuberculum sellae meningioma: Technical case report. Neurosurgery. 2007. 60: E401
31. Raper DM, Ding D, Evans E, Starke RM, Webster Crowley R, Liu KC. Clinical features, management considerations and outcomes in case series of patients with parasellar intracranial aneurysms undergoing anterior skull base surgery. World Neurosurg. 2017. 99: 424-32
32. Ren S, Lu Q, Xiao Y, Zhang Y, Zhang L, Li B. Coexistence of pituitary adenoma and primary pituitary lymphoma: A case report and review of the literature. Front Surg. 2022. 9: 842830
33. Revuelta R, Arriada-Mendicoa N, Ramirez-Alba J, Soto-Hernandez JL. Simultaneous treatment of a pituitary adenoma and an internal carotid artery aneurysm through a supraorbital keyhole approach. Minim Invasive Neurosurg. 2002. 45: 109-11
34. Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms. Stroke. 1998. 29: 251-6
35. Ruiz-Juretschke F, Iza B, Scola-Pliego E, Poletti D, Salinero E. Coincidental pituitary adenoma and planum sphenoidale meningioma mimicking a single tumor. Endocrinol Nutr. 2015. 62: 292-4
36. Seda L, Cukiert A, Nogueira KC, Huayllas MK, Liberman B. Intrasellar internal carotid aneurysm coexisting with GH-secreting pituitary adenoma in an acromegalic patient. Arq Neuropsiquiatr. 2008. 66: 99-100
37. Wakai S, Fukushima T, Furihata T, Sano K. Association of cerebral aneurysm with pituitary adenoma. Surg Neurol. 1979. 12: 503-7
38. WHO Classification of Tumours Editorial Boa, editors. World Health Organization classification of tumours of the central nervous system. Lyon: International Agency for Research on Cancer; 2021. p.
39. Xu K, Yuan Y, Zhou J, Yu J. Pituitary adenoma apoplexy caused by rupture of an anterior communicating artery aneurysm: Case report and literature review. World J Surg Oncol. 2015. 13: 228
40. Yamada K, Hatayama T, Ohta M, Sakoda K, Uozumi T. Coincidental pituitary adenoma and parasellar meningioma: Case report. Neurosurgery. 1986. 19: 267-70
41. Zhu H, Miao Y, Shen Y, Guo J, Xie W, Zhao S. Germline mutations in MEN1 are associated with the tumorigenesis of pituitary adenoma associated with meningioma. Oncol Lett. 2020. 20: 561-8
42. Zhu H, Miao Y, Shen Y, Guo J, Xie W, Zhao S. The clinical characteristics and molecular mechanism of pituitary adenoma associated with meningioma. J Transl Med. 2019. 17: 354