- Department of Radiology, Institute of Psychiatry and Neurology, Warsaw, Poland
- Department of Neurosurgery, Institute of Psychiatry and Neurology, Warsaw, Poland
- Department of Department of Medical Radiology, Military Institute of Medicine, Warsaw, Poland.
Correspondence Address:
Karol Sylwester Karamon, Department of Radiology, Institute of Psychiatry and Neurology, Warsaw, Poland. Sobieskiego street 9, 02-957 Warsaw, Poland.
DOI:10.25259/SNI_362_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Karol Sylwester Karamon1, Michał Sobstyl2, Marcin Rylski1, Katarzyna Wieczorek3. Sylvian fissure lipoma associated with fusiform aneurysm in the middle cerebral artery trifurcation: A case report and literature review. 28-Jul-2023;14:268
How to cite this URL: Karol Sylwester Karamon1, Michał Sobstyl2, Marcin Rylski1, Katarzyna Wieczorek3. Sylvian fissure lipoma associated with fusiform aneurysm in the middle cerebral artery trifurcation: A case report and literature review. 28-Jul-2023;14:268. Available from: https://surgicalneurologyint.com/surgicalint-articles/12462/
Abstract
Background: The intracranial lipomas are rare congenital malformations accounting for approximately 0.1–1.3% of all intracranial tumors, of which Sylvian fissure lipomas account for
Case Description: We present the case of a 42-year-old woman with chronic headaches and short-term memory impairment who was admitted to the emergency room after an out-of-hospital brain MRI with suspected ruptured right middle cerebral artery (MCA) aneurysm and late subacute intracranial hemorrhage. In the hospital, after clinical evaluation, emergency computed tomography (CT) angiography was performed, which revealed an unruptured fusiform aneurysm located in the right MCA trifurcation surrounded by an extremely hypodense lesion corresponding to fat in the right Sylvian fissure. No features of intracranial hemorrhage were present. The diagnosis of intracranial lipoma was finally confirmed after the MRI of the brain with a fat suppression sequence. Surgical treatment was not attempted, and the patient was treated conservatively with a satisfactory general outcome.
Conclusion: A Sylvian fissure lipoma may be associated with a fusiform aneurysm in the MCA trifurcation. By modifying the standard MRI protocol and performing a CT scan, an intracranial lipoma can be detected and a late subacute intracranial hemorrhage can be excluded.
Keywords: Aneurysm, Intracranial lipoma, Malformation, Middle cerebral artery, Sylvian fissure
INTRODUCTION
Intracranial lipomas are considered sporadic congenital malformations originating from abnormal fatty transformation and maldifferentiation of meninx primitiva at embryonic level.[
In specific MR sequences, lipomas due to its electromagnetic characteristics may mimic extracellular methemoglobin present in late subacute hemorrhage, contributing to challenging diagnostic issues, in particular when intracranial aneurysm is present.[
CASE REPORT
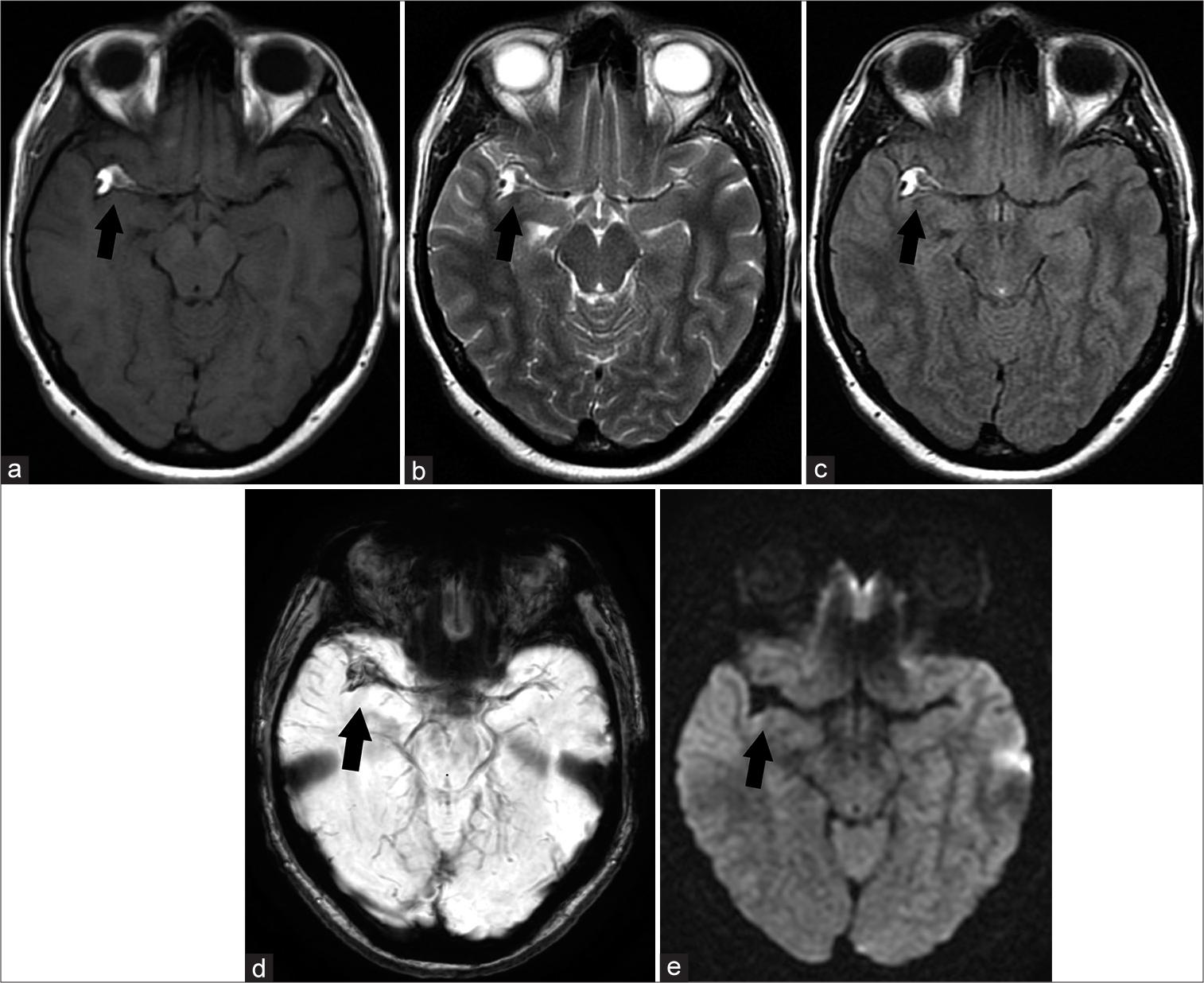
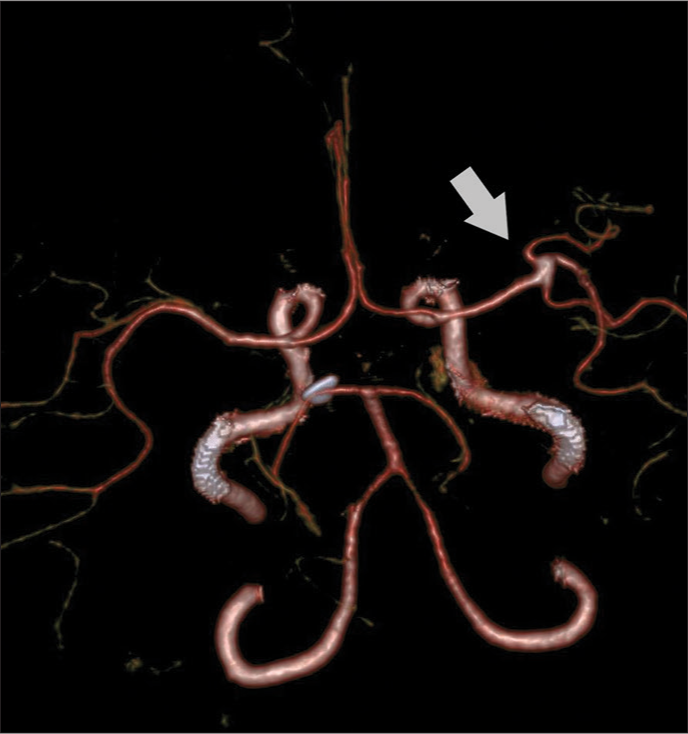
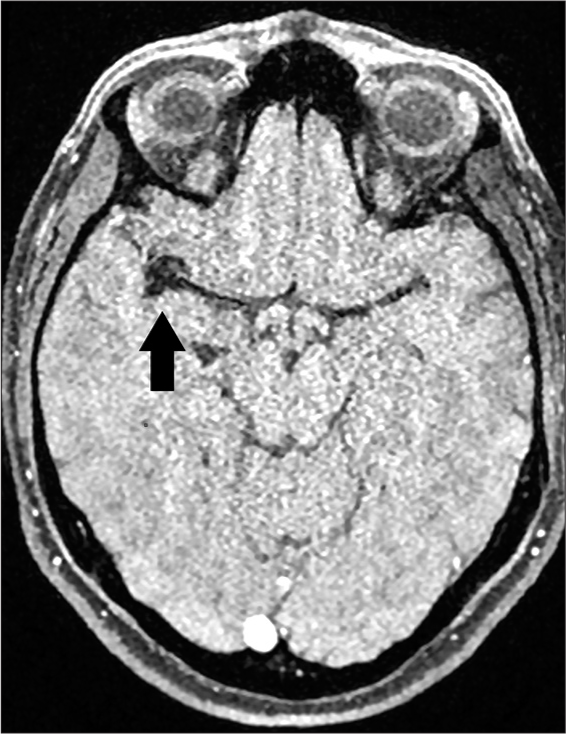
A 42 year-old woman with a chronic, periodically appearing headache lasting for the last few years treated successfully with analgesics and with short-term memory problems of unknown duration was referred to the emergency department by radiologist after out-of-hospital brain magnetic resonance imaging (MRI) with suspicion of right middle cerebral artery (MCA) aneurysm and late subacute subarachnoid hemorrhage in the right Sylvian fissure. It is essential to mention that the patient performed this study on her own and no previous clinical examination nor imaging study was conducted. On physical examination, her general condition was surprisingly good in relation to her initial diagnosis and showed moderate severity headache only. The rest of her medical and family history was unremarkable. In the meantime, the radiological consultation of the MRI provided by the patient was carried out. A lesion in the right Sylvian fissure with a dilated right MCA passing across it was visualized. The lesion was non-homogeneously hyperintense in T1W conventional spin echo (T1W CSE), T2W fast spin echo (T2W FSE), and fluid attenuated inversion recovery sequences (FLAIR). Moreover, gradient echo technique susceptibility weighted imaging (SWI) expressed hypointensity and blooming due to susceptibility artifacts. Diffusion-weighted imaging (DWI) did not show diffusion restriction [
Figure 1:
Non-contrast head MRI. Black arrow indicates right Sylvian fissure lipoma encasing right MCA. Axial scans show different signal intensity of the lesion in selected MRI sequences. (a) T1W CSE hyperintense lesion. (b) T2W FSE hyperintense lesion. (c) FLAIR hyperintense lesion. (d) SWI hypointense lesion with blooming artifacts. (e) DWI hypointense lesion. MRI: Magnetic resonance imaging, MCA: Middle cerebral artery, T1W CSE: T1W conventional spin echo, T2W FSE: T2W fast echo spin, SWI: Susceptibility weighted imaging, DWI Diffusion weighed imaging. FLAIR: Fluid-attenuated inversion recovery.
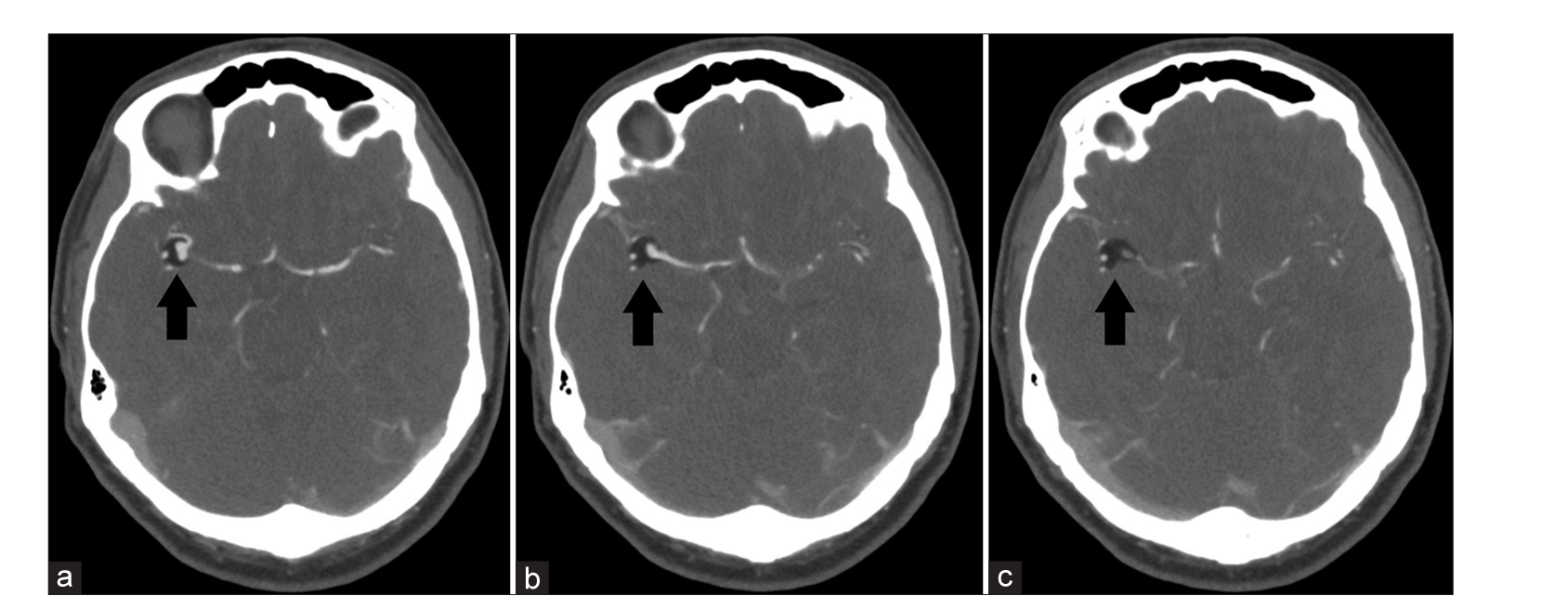
Figure 3:
Non-contrast head CT scan in axial projections showing 3 consecutive scans 2,5 mm apart. The black arrow on images (a-c) show a cross-section at different levels of the lesion located in the right Sylvian fissure corresponding to a lipoma and encasing the right MCA. CT: Computed tomography, MCA: Middle cerebral artery.
DISCUSSION
Intracranial lipomas are rare lesions with the prevalence of 0.1–1.3%.[
Intracranial lipomas are found at any age, mostly incidentally, usually unrelated directly with primary complaint.[
Other less common symptoms include vomiting, hemiplegia, vertigo, mental retardation, emotional lability, and cranial nerve impairment.[
Depending on the source, about 25–60% of the intracranial lipomas are associated with dysgenesis of neuronal brain tissues and vascular malformations.[
Aneurysm formation in association with lipoma does not appear to be incidental; however, the biological mechanism is unclear.[
Intracranial lipomas may mimic late subacute hemorrhage due to similar radiological features on MR-based imaging and this may lead to misdiagnosis. Both these entities present short T1W and long T2W times expressed as hyperintense signal. Furthermore, in these conditions, gradient echo technique SWI shows hypointensity and blooming artifacts, due to its specific modality in the detection deposits of diamagnetic and paramagnetic substances.[
Bakshi et al. came up with the solution of how differentiation between lipoma and late subacute hemorrhage could be achieved.[
On CT, intracranial lipomas appear as non-enhancing mass with very low density ranging between −40 and −100 HU with sporadic calcifications present and no similarities to blood products.[
MR of the head is often the diagnostic test of choice for chronic non-traumatic headache.[
Treatment of intracranial lipomas depends on the size and location as well as the presence of symptoms and complications. For the vast majority of intracranial lipomas, the management is based on the simple monitoring of the patient’s condition and when symptomatic conservative treatment is recommended. Surgery is limited to very specific conditions, in which conservative treatment is not effective. Attempts at gross total lipoma resection have a relatively high risk of brain injury due to the abundant vascularization of the lesion and the tight adhesion of the lipoma to surrounding nerve structures and vessels.[
Symptomatic lipomas of Sylvian fissure associated with vascular malformation of the MCA or its branches are in most cases treated conservatively, and operational approach is rarely applied. The literature describes several cases of different surgical approaches to such complicated intracranial lipomas with generally satisfactory outcomes.[
In the presented case, after a complex neurological and neurosurgical consultation, a decision was made to treat the patient conservatively. Closed follow-up with a scheduled angio-RM and angio-CT was ordered to observe the potential progression of the fusiform aneurysm over time. In this case, the risks associated with brain damage far outweighed the benefits of surgery.
CONCLUSION
The case we present shows that the lipoma can be located in the Sylvian fissure in close proximity to the MCA. The intracranial lipoma can coexist with vascular abnormalities, such as the fusiform aneurysm in the trifurcation of the right MCA. In contrast to lipoma in the pericallosal cistern, lipoma in the Sylvian fissure appears to be more often associated with a saccular aneurysm than with a fusiform aneurysm. Special attention should be given to appropriate modification of the MRI protocol and the performance of a head CT that can establish a definitive diagnosis by ruling out late subacute hemorrhage. The decision on the treatment modality should be made by a comprehensive clinical evaluation of the patient’s condition, and surgical treatment should be considered only for symptomatic patients after a prior assessment of its risks and benefits.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
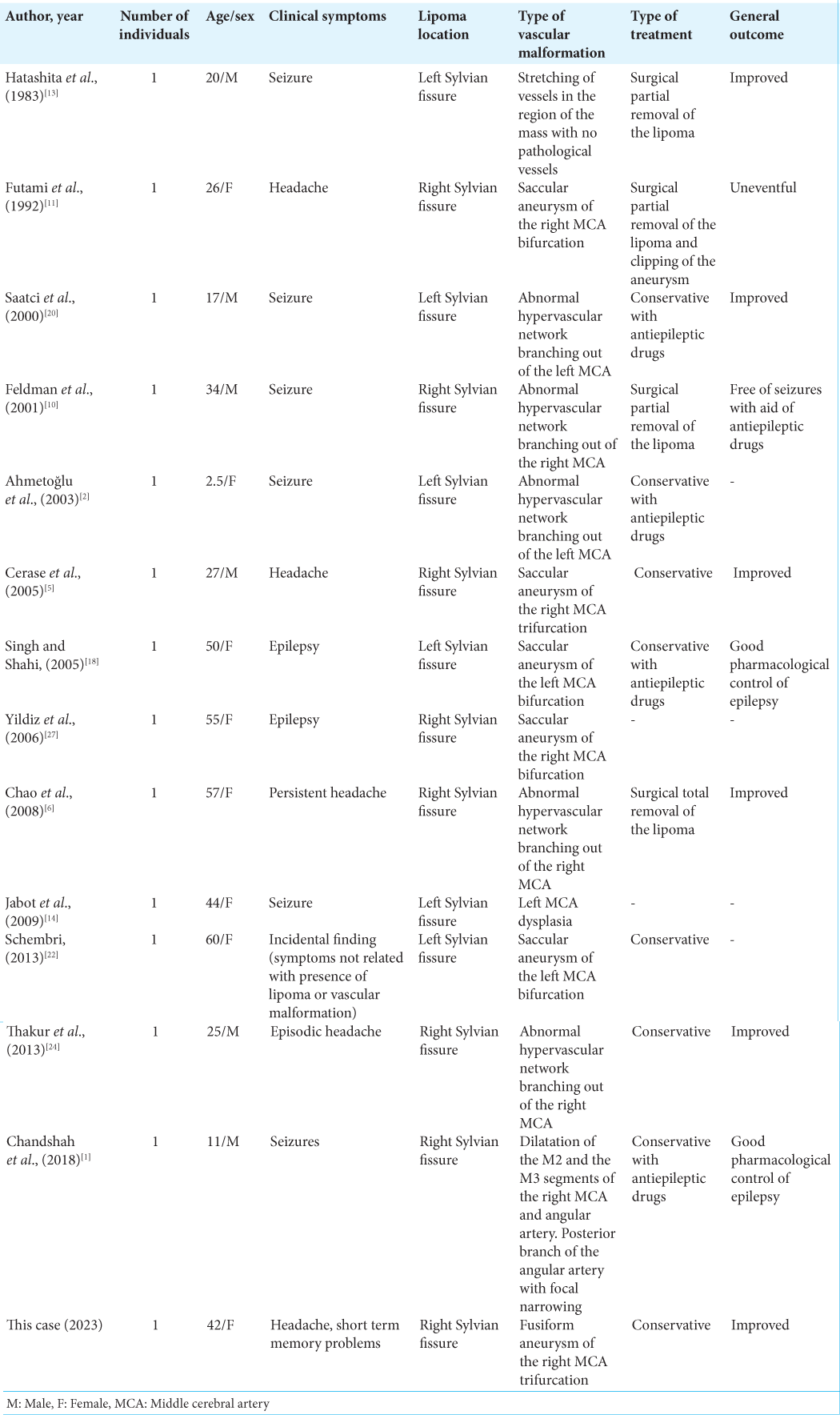
1. Ahmed Chandshah MI, Sadashiva N, Saini J, Shukla D. Distal Sylvian fissure lipoma masquerading as a vascular malformation with bleed. Neurol India. 2018. 66: 1518-20
2. Ahmetoğlu A, Aynaci FM, Sari A. Sylvian fissure lipoma associated with cortical dysplasia and abnormal vascularity. Eur J Radiol Extra. 2003. 46: 43-6
3. Bakshi R, Shaikh ZA, Kamran S, Kinkel PR. MRI findings in 32 consecutive lipomas using conventional and advanced sequences. J Neuroimaging. 1999. 9: 134-40
4. Brzegowy P, Polak J, Wnuk J, Łasocha B, Walocha J, Popiela TJ. Middle cerebral artery anatomical variations and aneurysms: A retrospective study based on computed tomography angiography findings. Folia Morphol (Warsz). 2018. 77: 434-40
5. Cerase A, Vallone IM, Atesalp A, Demirci A, Venturi C, Vural M. Intracranial aneurysm associated with perianeurysmal fat tissue lesion and parenchymal cystic changes. Rivista di Neuroradiologia. 2005. 18: 575-80
6. Chao SC, Shen CC, Cheng WY. Microsurgical removal of sylvian fissure lipoma with pterion keyhole approach-case report and review of the literature. Surg Neurol. 2008. 70: 85-90
7. De Luca GC, Bartleson JD. When and how to investigate the patient with headache. Semin Neurol. 2010. 30: 131-44
8. Eghwrudjakpor PO, Kurisaka M, Fukuoka M, Mori K. Intracranial lipomas: Current perspectives in their diagnosis and treatment. Br J Neurosurg. 1992. 6: 139-44
9. Eldevik OP, Gabrielsen TO. Fusiform aneurysmal dilatation of pericallosal artery. A sign of lipoma of corpus callosum. Acta Radiol Suppl. 1976. 347: 71-6
10. Feldman RP, Marcovici A, LaSala PA. Intracranial lipoma of the sylvian fissure. Case report and review of the literature. J Neurosurg. 2001. 94: 515-9
11. Futami K, Kimura A, Yamashita J. Intracranial lipoma associated with cerebral saccular aneurysm. Case report. J Neurosurg. 1992. 77: 640-2
12. Gossner J. Small intracranial lipomas may be a frequent finding on computed tomography of the brain. A case series. Neuroradiol J. 2013. 26: 27-9
13. Hatashita S, Sakakibara T, Ishii S. Lipoma of the insula. Case report. J Neurosurg. 1983. 58: 300-2
14. Jabot G, Stoquart-Elsankari S, Saliou G, Toussaint P, Deramond H, Lehmann P. Intracranial lipomas: Clinical appearances on neuroimaging and clinical significance. J Neurol. 2009. 256: 851-5
15. Kazner E, Stochdorph O, Wende S, Grumme T. Intracranial lipoma. Diagnostic and therapeutic considerations. J Neurosurg. 1980. 52: 234-45
16. Lingegowda D, Rajashekar C, Belaval VV, Thomas B, Keshavdas C, Kapilamoorthy . Susceptibility artifacts in lipomas. Neurol India. 2013. 61: 56-9
17. Maiuri F, Cirillo S, Simonetti L. Lipoma of the sylvian region. Clin Neurol Neurosurg. 1989. 91: 321-3
18. Pal Singh G, Rai Shahi J. Sylvian fissure lipoma with aneurysm of middle cerebral arterty-a case report. Ann Indian Acad Neurol. 2005. 8: 323-6
19. Peter AB, Ramli N, Rahmat K, Rozalli FI, Azlan CA. Mimics and Diagnostic Pitfalls of Intracranial Lesions in Conventional MRI: Clues on advanced MRI. Neurology Asia. 2015. 20: 161-5
20. Saatci I, Aslan C, Renda Y, Besim A. Parietal lipoma associated with cortical dysplasia and abnormal vasculature: case report and review of the literature. AJNR Am J Neuroradiol. 2000. 21: 1718-21
21. Sarioğlu AC, Kaynar MY, Hanci M, Uzan M. Sylvian fissure lipomas: Case reports and review of the literature. Br J Neurosurg. 1999. 13: 386-8
22. Schembri N. Sylvian fissure lipoma associated with middle cerebral artery aneurysm-report of a rare case highlighting imaging pitfalls. Int Neuropsychiatr Dis J. 2013. 1: 16-23
23. Taglialatela G, Galasso R, Taglialatela G, Conforti R, Volpe A, Galasso L. Lipomas of corpus callosum. Neuroanatomy. 2009. 8: 39-42
24. Thakur S, Sood RG, Jhobta A, Makhaik S, Thakur C. Sylvian fissure lipoma with angiomatous component and associated brain malformation: A case report. Iran J Neurol. 2013. 12: 169-71
25. Truwit CL, Barkovich AJ. Pathogenesis of intracranial lipoma: An MR study in 42 patients. AJNR Am J Neuroradiol. 1990. 11: 665-74
26. Wallace D. Lipoma of the corpus callosum. J Neurol Neurosurg Psychiatry. 1976. 39: 1179-85
27. Yildiz H, Hakyemez B, Koroglu M, Yesildag A, Baykal B. Intracranial lipomas: Importance of localization. Neuroradiology. 2006. 48: 1-7