- Department of Neurosurgery, Saint Louis University, 3635 Vista Avenue, St, Louis, Missouri, United States.
DOI:10.25259/SNI_257_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Maheen Qamar Khan, Cristian Cirjan, Nabiha Quadri, Georgios Alexopoulos, Jeroen Coppens. Symptomatic cerebral vasospasm in the setting of carmustine wafer placement for glioblastoma: A case presentation and review of literature. 27-Jun-2020;11:168
How to cite this URL: Maheen Qamar Khan, Cristian Cirjan, Nabiha Quadri, Georgios Alexopoulos, Jeroen Coppens. Symptomatic cerebral vasospasm in the setting of carmustine wafer placement for glioblastoma: A case presentation and review of literature. 27-Jun-2020;11:168. Available from: https://surgicalneurologyint.com/surgicalint-articles/10104/
Abstract
Background: Gliadel placement in glioblastoma resection, particularly with concurrent chemoradiation, has demonstrated an improvement in survival. There have been several reported adverse effects, some of which lend to significantly increased morbidity and mortality. With only two other cases described in literature, cerebral vasospasm secondary to carmustine-impregnated wafers is an extremely rare side effect.
Case Description: We report the case of a 51-year-old female who presented with the left lower limb paresis 8 days after high-grade glioma resection provoked by carmustine wafer placement.
Conclusion: We urge surgeons to reconsider placement of carmustine wafers in nations where the surgical resection cavity includes exposed large cerebral vasculature. We also propose the early identification of this devastating complication in the postoperative period by maintaining a high clinical suspicion and prompt utilization of computed tomography and digital subtraction angiography in the management and treatment of these patients accordingly.
Keywords: Carmustine wafer, Cerebral vasospasm, Glial tumor, Glioma, Tumor resection
BACKGROUND
Carmustine-impregnated wafers (Gliadel®, Eisai, Baltimore, Maryland, USA) are biodegradable polymers designed to deliver high concentrations of 1,3-bis-2-chloroethyl-1-nitrosourea (BCNU) into cerebral tumor resection cavities while surpassing the blood-brain barrier and avoiding the constitutional side effects of systemic chemotherapy.[
CASE REPORT
A 51-year-old female was transferred to our institution after the discovery of a large right temporal and insular lesion on magnetic resonance imaging (MRI). The patient initially presented with a few months of headaches and smelling odd odors to her primary care physician, who then prescribed the patient levetiracetam (Keppra, UCB Pharmaceuticals, Belgium) and referred her to our institution. The patient had no deficits on gross physical examination. Contrasted computed tomography (CT) with contrast revealed a large mass with irregular enhancement and MRI revealed moderate perilesional edema with mass effect [
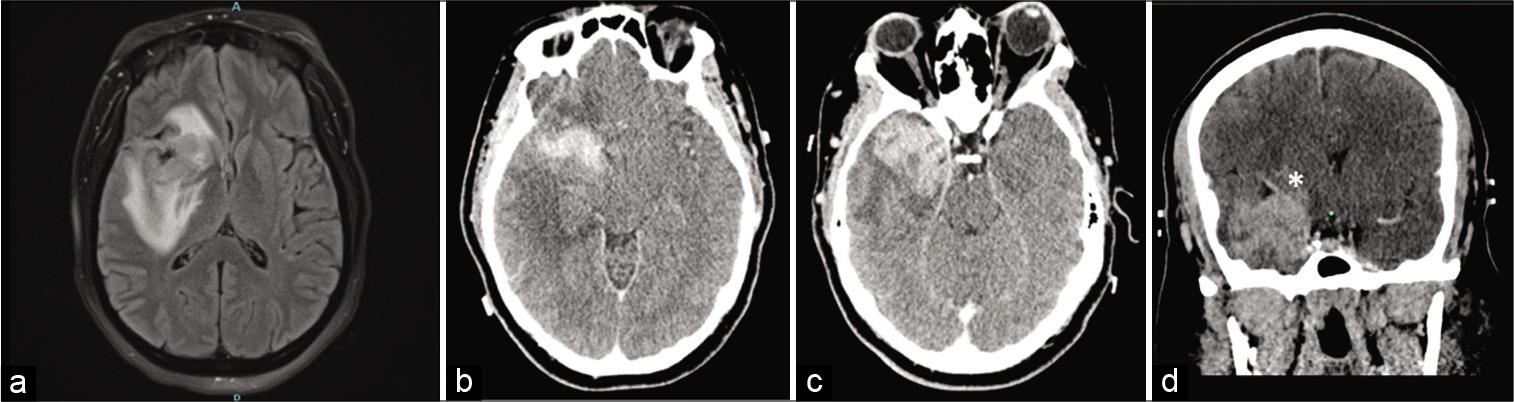
Figure 1:
(a) FLAIR magnetic resonance imaging showing right temporal and insular lesion with right to left mass effect and surrounding edema. (b and c) CT with contrast demonstrating irregular enhancement of a large right temporal and insular lesion. (d) A coronal view of the preoperative CT with contrast, with an emphasis on the Sylvian fissure and middle cerebral artery (MCA) being pushed upward (asterisk).
The patient underwent an uncomplicated right frontotemporal craniotomy for tumor resection. At the anteromedial extent of the tumor, the Sylvian vessels were encountered and preserved. The dissection of the tumor was performed with preservation of the pial demarcation of the Sylvian fissure. The anterior choroidal artery and MCA branches were met and not manipulated as this indicated the anterior extent of the tumor. An amygdalohippocampectomy was also performed. Intraoperative monitoring, with somatosensory evoked potentials, motor evoked potentials, and electroencephalography, was performed throughout the course of the operation and remained at baseline. Frozen pathology confirmed the presence of high-grade glioma, and at that time, the decision was made to line the surgical cavity, including exposed Sylvian fissure and large cerebral vessels, with four carmustine wafers. Two wafers were placed along the cephalad resection cavity in direct contact with the exposed portion of the Sylvian fissure and the remainder along the posterior resection wall [
Postoperatively, the patient experienced no worsening deficits. Immediate postoperative CT revealed small volume hemorrhage within the posteromedial aspects of the resection cavity, measured to be about 1.7 cm3 per volumetric analysis through OsiriX software (Apple, California) [
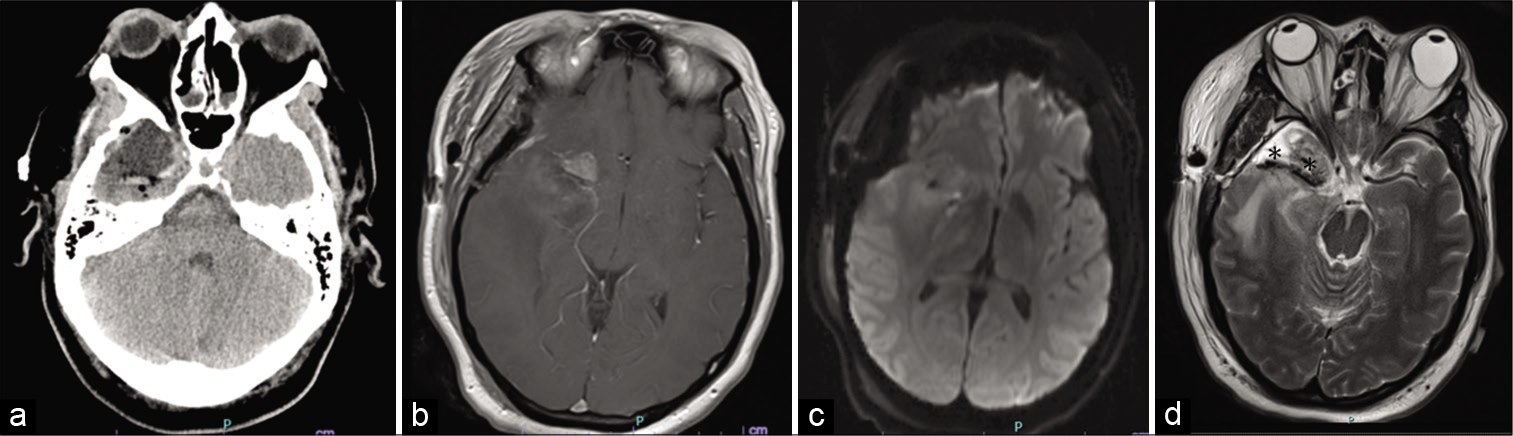
Figure 2:
(a) Postoperative CT with minimal hemorrhage in the posterior aspect of the resection cavity. (b) The T1 magnetic resonance imaging (MRI) with contrast respectively, with small amount of residual tumor anterosuperiorly and medially, with small amount of hemorrhage within the resection cavity and no evidence of hemorrhage within the basal cisterns. (c) Diffusion-weighted imaging without any evidence of infarct. (d) A T2 MRI with hypointensities along exposed middle cerebral artery (asterisks), representing Gliadel wafer lining the vessel and cavity.
On postoperative day 8, the patient experienced new left lower extremity weakness, which progressed to include slurring of speech and left facial weakness. Over the course of the next 24 h, this evolved to plegia in the left upper extremity and severe hemiparesis in the left lower extremity. MRI revealed stable edema and an acute ischemic stroke in the right posterior limb of the internal capsule [
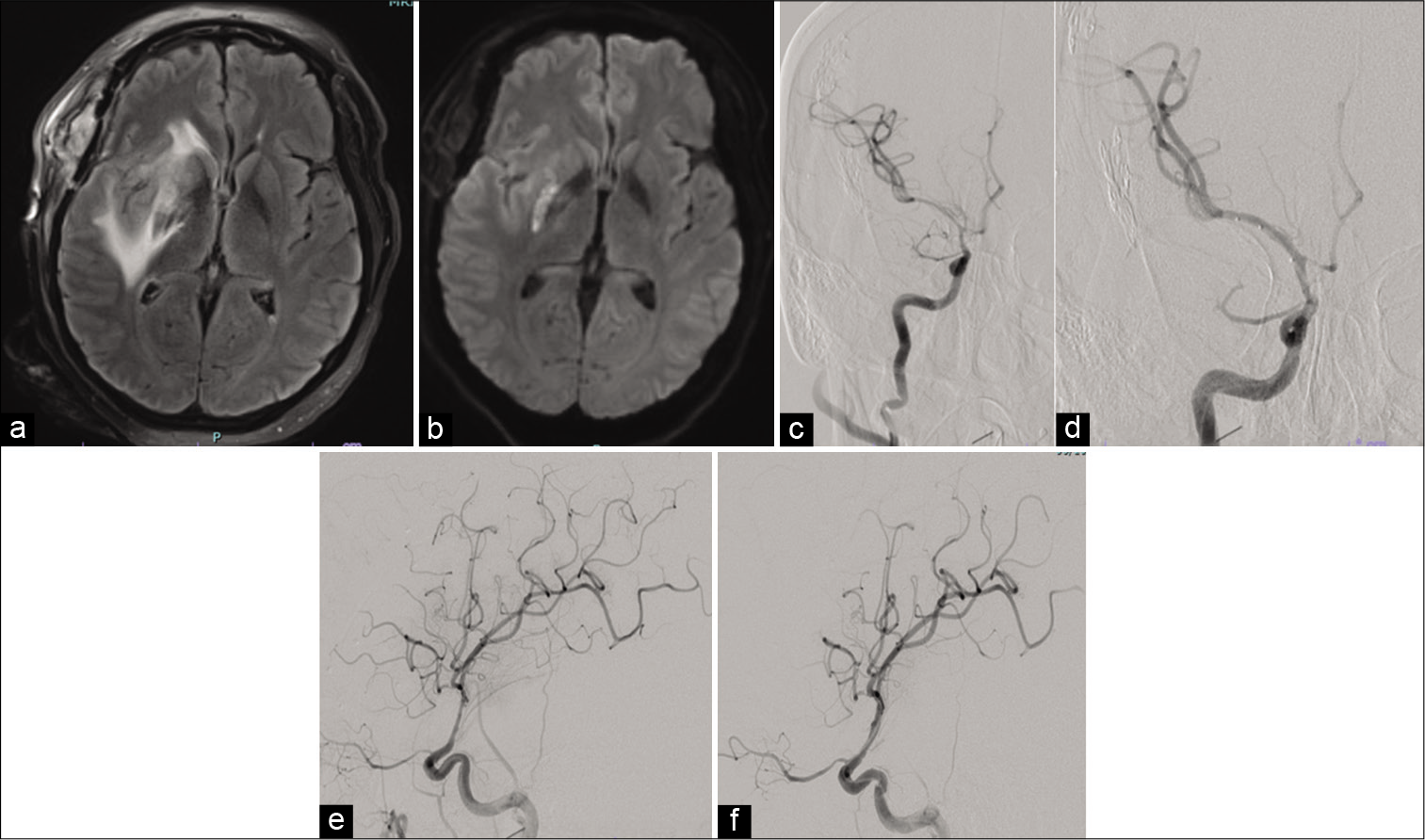
Figure 3:
(a) FLAIR magnetic resonance imaging revealing stable perilesional edema on postoperative day 8. (b) Diffusion-weighted imaging showcasing a right posterior limb of the internal capsule acute infarct. (c-f) Cerebral angiographic images of the anterior circulation displaying moderate vasospasm in the right supraclinoid internal carotid artery (ICA), the carotid terminus, A1 of the anterior cerebral artery, and M1 of the middle cerebral artery (MCA). (e and f) Improvement in caliber and diameter of supraclinoid ICA and MCA after angioplasty and intra-arterial injection of nicardipine.
DISCUSSION
CVS is a well-described entity in patients with aneurysmal subarachnoid hemorrhage (aSAH)[
This case does not include the aforementioned factors. There was no manipulation of exposed Sylvian vessels, given that this was the anterior extent of our resection. The vessels were identified and preserved. The delayed timing of the sCVS does not match the course of “traction hemiplegia,” which tends to manifest as CVS intraoperatively or immediately in the perioperative course and is short termed.[
Gliadel polymers function through controlled release of contents over a period of 2–3 weeks after implantation.[
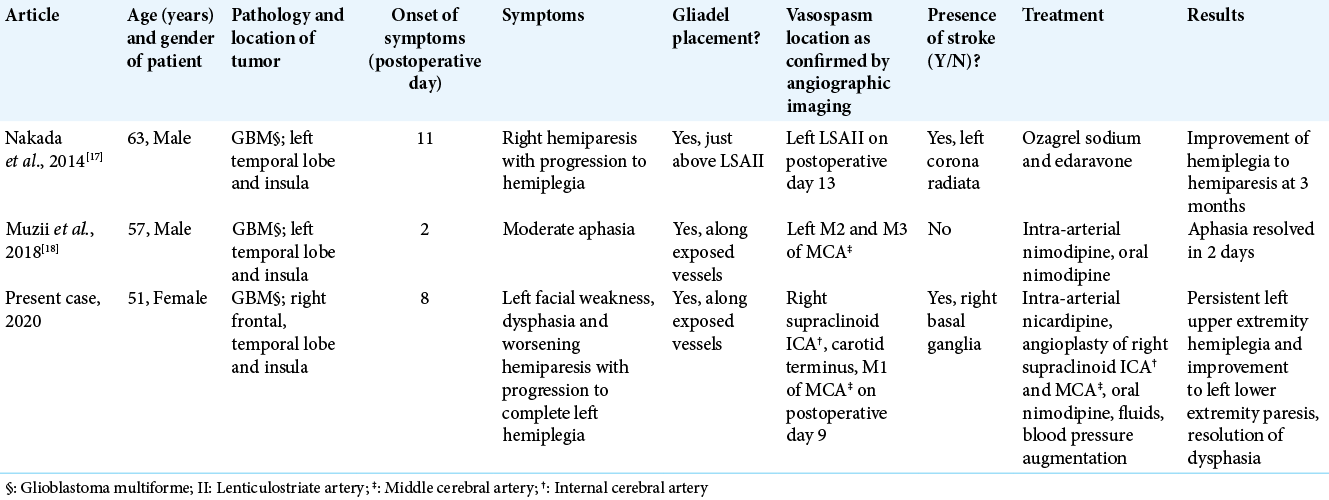
The previous cases of sCVS with Gliadel implantation along with ours are outlined in [
In all the cases of sCVS with Gliadel implantation [
Treatment of sCVS is similar to that instituted in aSAH.[
Given that Gliadel does lend a survival benefit in a devastating disease,[
CONCLUSION
We urge surgeons to reconsider universal placement of carmustine wafers in GBM in situations where large intracranial vasculature is exposed within the tumor resection cavity. We also propose the early identification of this devastating complication in the postoperative period by maintaining a high clinical suspicion and prompt utilization of CT and DSA in the management and treatment of these patients accordingly.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alotaibi NM, Lanzino G. Cerebral vasospasm following tumor resection. J Neurointerv Surg. 2013. 5: 413-8
2. Aoki T, Nishikawa R, Sugiyama K, Nonoguchi N, Kawabata N, Mishima K. A multicenter phase I/II study of the BCNU implant (gliadel® wafer) for Japanese patients with malignant gliomas. Neurol Med Chir (Tokyo). 2014. 54: 290-301
3. Ashby LS, Smith KA, Stea B. Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: A systematic literature review. World J Surg Oncol. 2016. 14: 1-15
4. Bauer AM, Rasmussen PA. Treatment of intracranial vasospasm following subarachnoid hemorrhage. Front Neurol. 2014. 5: 1-7
5. Bejjani GK, Sekhar LN, Yost AM, Bank WO, Wright DC. Vasospasm after cranial base tumor resection: Pathogenesis, diagnosis, and therapy. Surg Neurol. 1999. 52: 577-84
6. Champeaux C, Weller J. Implantation of carmustine wafers (Gliadel®) for high-grade glioma treatment. A 9-year nationwide retrospective study. J Neurooncol. 2020. 147: 159-69
7. Chang SD, Yap OW, Adler JR. Symptomatic vasospasm after resection of a suprasellar pilocytic astrocytoma: Case report and possible pathogenesis. Surg Neurol. 2015. 51: 521-7
8. Charpentier C, Audibert G, Guillemin F, Civit T, Ducrocq X, Bracard S. Multivariate analysis of predictors of cerebral vasospasm occurrence after aneurysmal subarachnoid hemorrhage. Stroke. 1999. 30: 1402-8
9. Chowdhary SA, Ryken T, Newton HB. Survival outcomes and safety of carmustine wafers in the treatment of high-grade gliomas: A meta-analysis. J Neurooncol. 2015. 122: 367-82
10. Claassen J, Bernardini GL, Kreiter K, Bates J, Du YE, Copeland D. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: The fisher scale revisited. Stroke. 2001. 32: 2012-20
11. Dickerson JC, Hidalgo JA, Smalley ZS, Shiflett JM. Diffuse vasospasm after transcortical temporal lobectomy for intractable epilepsy. Acta Neurochir (Wien). 2018. 160: 1883-7
12. Duntze J, Litré CF, Eap C, Théret E, Debreuve A, Jovenin N. Implanted carmustine wafers followed by concomitant radiochemotherapy to treat newly diagnosed malignant gliomas: Prospective, observational, multicenter study on 92 cases. Ann Surg Oncol. 2013. 20: 2065-72
13. Fleming AB, Saltzman WM. Pharmacokinetics of the carmustine implant. Clin Pharmacokinet. 2002. 41: 403-19
14. Fung LK, Ewend MG, Sills A, Sipos EP, Thompson R, Watts M. Pharmacokinetics of interstitial delivery of carmustine, 4-hydroperoxycyclophosphamide, and paclitaxel from a biodegradable polymer implant in the monkey brain. Cancer Res. 1998. 58: 672-84
15. Gutenberg A, Lumenta CB, Braunsdorf WE, Sabel M, Mehdorn HM, Westphal M. The combination of carmustine wafers and temozolomide for the treatment of malignant gliomas. A comprehensive review of the rationale and clinical experience. J Neurooncol. 2013. 113: 163-74
16. Hart MG, Garside R, Rogers G, Somerville M, Stein K, Grant R. Chemotherapy wafers for high grade glioma. Cochrane Database Syst Rev. 2011. 3: CD007294-
17. Inada M, Shindo M, Kobayashi K, Sato A, Yamamoto Y, Akasaki Y. Anticancer effects of a non-narcotic opium alkaloid medicine, papaverine, in human glioblastoma cells. PLoS One. 2019. 14: e0216358-
18. Kuramitsu S, Motomura K, Natsume A, Wakabayashi T. Double-edged sword in the placement of carmustine (BCNU) wafers along the eloquent area: A case report. NMC Case Rep J. 2015. 2: 40-5
19. Lackner P, Koppelstaetter F, Ploner P, Sojer M, Dobesberger J, Walser G. Cerebral vasospasm following temporal lobe epilepsy surgery. Neurology. 2012. 78: 1215-20
20. Mandonnet E, Chassoux F, Naggara O, Roux FX, Devaux B. Transient symptomatic vasospasm following antero-mesial temporal lobectomy for refractory epilepsy. Acta Neurochir (Wien). 2009. 151: 1723-6
21. Muzii VF, Vaiano A, Bracco S, Carangelo BR. Symptomatic cerebral vasospasm after glioblastoma resection and carmustine wafers implantation. A case report. Interdiscip Neurosurg Adv Tech Case Manag. 2018. 14: 24-7
22. Nakada M, Tanaka S, Oishi M, Miyashita K, Misaki K, Mohri M. Cerebral infarction related to carmustine wafers in glioblastoma: A case report. NMC Case Rep J. 2014. 2: 36-9
23. Rahmani P, Rezvani M, Sabouri M, Nikbakht H, Rafiee A, Torkashvand M. The effect of irrigation of intracisternal papaverine on cerebral blood flow in subarachnoid hemorrhage. Adv Biomed Res. 2013. 2: 45-
24. Rao S, Narayanan S, Nanjireddy R, Mittal S, Basha M. Pearls and oy-sters: Symptomatic cerebral vasospasm on conventional angiography following temporal lobe epilepsy surgery. Neurology. 2017. 88: e230-2
25. Rath GP, Mukta , Prabhakar H, Dash HH, Suri A. Haemodynamic changes after intracisternal papaverine instillation during intracranial aneurysmal surgery. Br J Anaesth. 2006. 97: 848-50
26. Sampath P, Brem H. Implantable slow-release chemotherapeutic polymers for the treatment of malignant brain tumors. Cancer Control. 1998. 5: 130-7
27. Sato K, Dan M, Yamamoto D, Miyajima Y, Hara A, Kumabe T. Chronic phase intracranial hemorrhage caused by ruptured pseudoaneurysm induced by carmustine wafer implantation for insulo-opercular anaplastic astrocytoma: A case report. Neurol Med Chir (Tokyo). 2015. 55: 848-51
28. Schaller C, Zentner J. Vasospastic reactions in response to the transsylvian approach. Surg Neurol. 1998. 49: 170-5
29. Schaller C, Haun D, Schramm J, Meyer B. Technique assessment the transsylvian approach is. “minimally invasive” but not atraumatic. Neurosurgery. 2002. 51: 971-7
30. Seeger W, Suttorp N, Schmidt F, Neuhof H. The glutathione redox cycle as a defense system against hydrogen-peroxide-induced prostanoid formation and vasoconstriction in rabbit lungs. Am Rev Respir Dis. 1986. 133: 1029-36
31. Shibahara I, Hanihara M, Watanabe T, Dan M, Sato S, Kuroda H. Tumor microenvironment after biodegradable BCNU wafer implantation: Special consideration of immune system. J Neurooncol. 2018. 137: 417-27
32. Shingleton BJ, Albert DM, Bienfang DC, Ensminger WD, Chandler WF, Greenberg HS. Ocular toxicity associated with high-dose carmustine. Arch Ophthalmol. 1982. 100: 1766-72
33. Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC. A Phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2004. 5: 79-88
34. Woo PY, See KW, Chow JK, Chan Y, Wong HT, Chan KY. Hypertensive-nimodipine therapy for middle cerebral artery vasospasm after resection of glioblastoma multiforme: A case report and literature review. Open J Mod Neurosurg. 2015. 5: 76-83
35. Zhang JH, Pluta RM, Hansen-Schwartz J, Dreier J, Vajkoczy P, Macdonald RL. Cerebral vasospasm following subarachnoid hemorrhage: Time for a new world of thought. Neurol Res. 2009. 31: 151-8