- Department of Medical Education, College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, Saudi Arabia.
- Departments of Pathology and Laboratory Medicine, King Abdulaziz Medical City, Ministry of National Guard Health Affairs, Jeddah, Makkah, Saudi Arabia.
- Departments of Radiology King Abdulaziz Medical City, Ministry of National Guard Health Affairs, Jeddah, Makkah, Saudi Arabia.
- Departments of Surgery, King Abdulaziz Medical City, Ministry of National Guard Health Affairs, Jeddah, Makkah, Saudi Arabia.
Correspondence Address:
Fayez Dhafer Alshehri
Departments of Surgery, King Abdulaziz Medical City, Ministry of National Guard Health Affairs, Jeddah, Makkah, Saudi Arabia.
DOI:10.25259/SNI_429_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Fayez Dhafer Alshehri1, Salem Khaled Baeshen1, Alaa Mohammed Noor Samkari2, Abeer Salim Almehdar3, Ahmed Ibrahim Lary4. Synovial sarcoma of the spine: A case report and review of the literature. 21-Aug-2020;11:257
How to cite this URL: Fayez Dhafer Alshehri1, Salem Khaled Baeshen1, Alaa Mohammed Noor Samkari2, Abeer Salim Almehdar3, Ahmed Ibrahim Lary4. Synovial sarcoma of the spine: A case report and review of the literature. 21-Aug-2020;11:257. Available from: https://surgicalneurologyint.com/surgicalint-articles/10219/
Abstract
Background: Synovial sarcoma (SS) of the spine is a rare malignant soft-tissue tumor, and there are few reported cases. The aim of this paper is to report a rare case of spinal SS involving the paraspinal muscles, and to review all such cases reported in the literature.
Case Description: In this paper, we report a rare case of spinal SS involving the paraspinal muscles in a 12-year-old girl. The patient underwent surgical excision of the mass with adjuvant radiation and chemotherapy. At the 1-year follow-up, there was no evidence of local tumor recurrence, and the patient’s symptoms had improved. In addition, we identified and reviewed 33 reported cases of SS involving the spine.
Conclusion: Due to the limited number of reported cases in the literature, it is difficult to predict the outcomes of spinal SS. Further, different treatment modalities have been used to treat spinal SS. However, most of the reported cases had poor outcomes. Therefore, prospective multi-center studies are needed to further investigate the treatment strategies and outcomes for patients with spinal SS.
Keywords: Hemangiopericytoma, Paraspinal, Spinal synovial sarcoma, Spinal tumor, Spinal tumors
INTRODUCTION
Synovial sarcoma (SS) is a rare malignant soft--tissue tumor accounting for 5–10% of all soft- tissue tumors.[
CASE DESCRIPTION
A 12-year-old female presented to our neurosurgery clinic with mid-lower back pain radiating to the left frontal aspects of the thigh. Four months before the presentation, the patient discovered a lump in the lower aspect of her back. The lump was small and increased in size. Her medical and surgical history was unremarkable. Physical examination revealed a large paravertebral lump (~4 × 5 cm) in the mid-lower aspect of the back. The lump was tender to light palpation and hard inconsistency, with irregular borders and no skin changes or muscle atrophy. Neurological examination of the lower limbs revealed normal tone, power, and intact sensation. Imaging studies (spinal magnetic resonance imaging [MRI] and computed tomography [CT]) revealed a left paraspinal soft-tissue mass extending from the T12–L1 level to the L4 vertebral level [
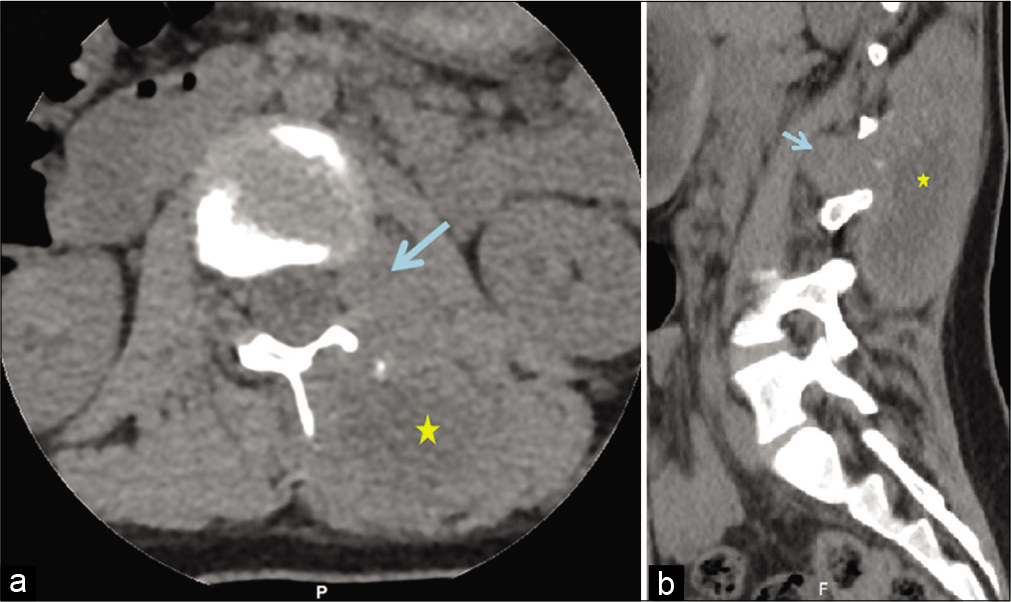
Figure 1:
Selected computed tomography images of the spine. Axial (a) and sagittal (b) views demonstrate the left paraspinal soft-tissue mass (*) with an area of calcification that is extended from the level of T12/L1 down to the L4 level vertebra. Note the bone erosion changes in the lamina at the axial plane and the extension into the L1/L2 left neural foramina (arrows).
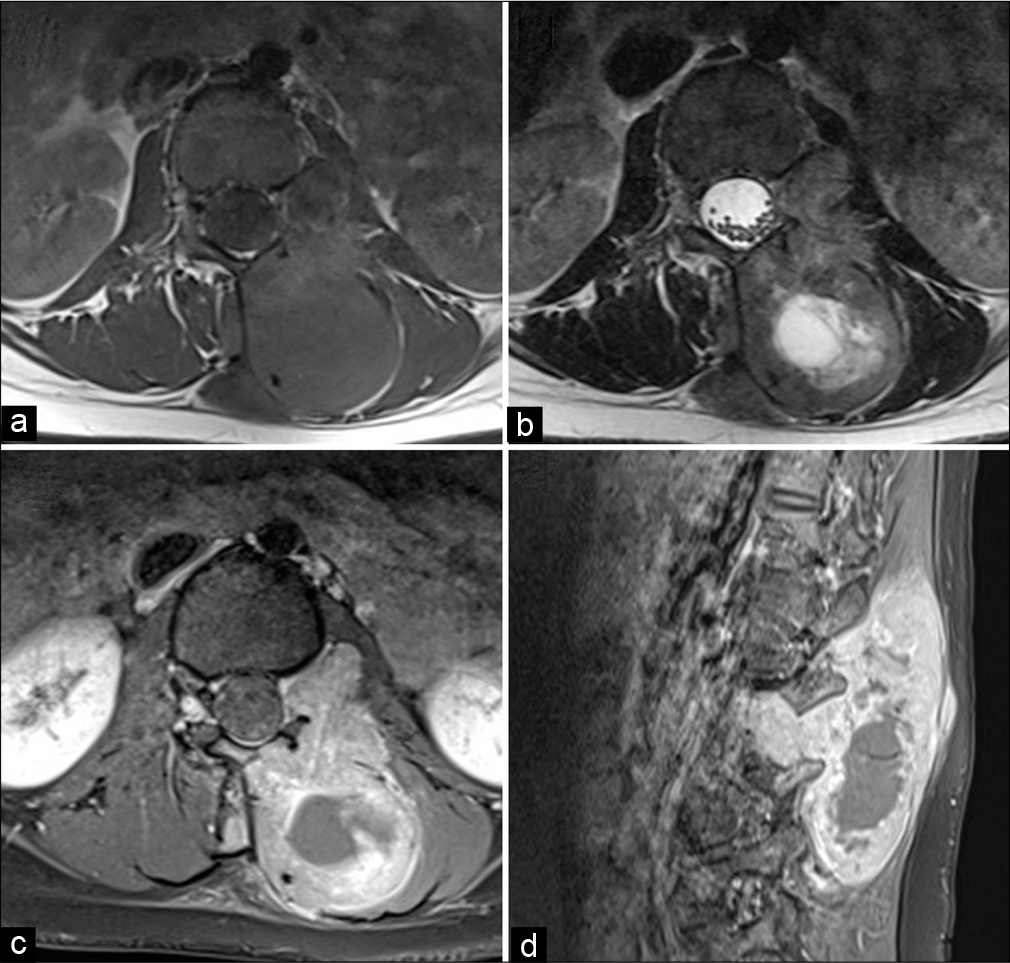
Figure 2:
Selected magnetic resonance images of the lumbosacral spine. Axial T1- and T2-weighted images at the L1/L2 level in the left neural foramina (a and b) demonstrate the left paraspinal soft- tissue mass causing erosion of the left side lamina and the spinous process of the L2 vertebra with extension through the neural foramina. No involvement of the cauda equina terminal nerve roots is noted. Axial and Sagittal T1 FS-weighted images (c and d) at the same level demonstrate soft-tissue mass enhancement.
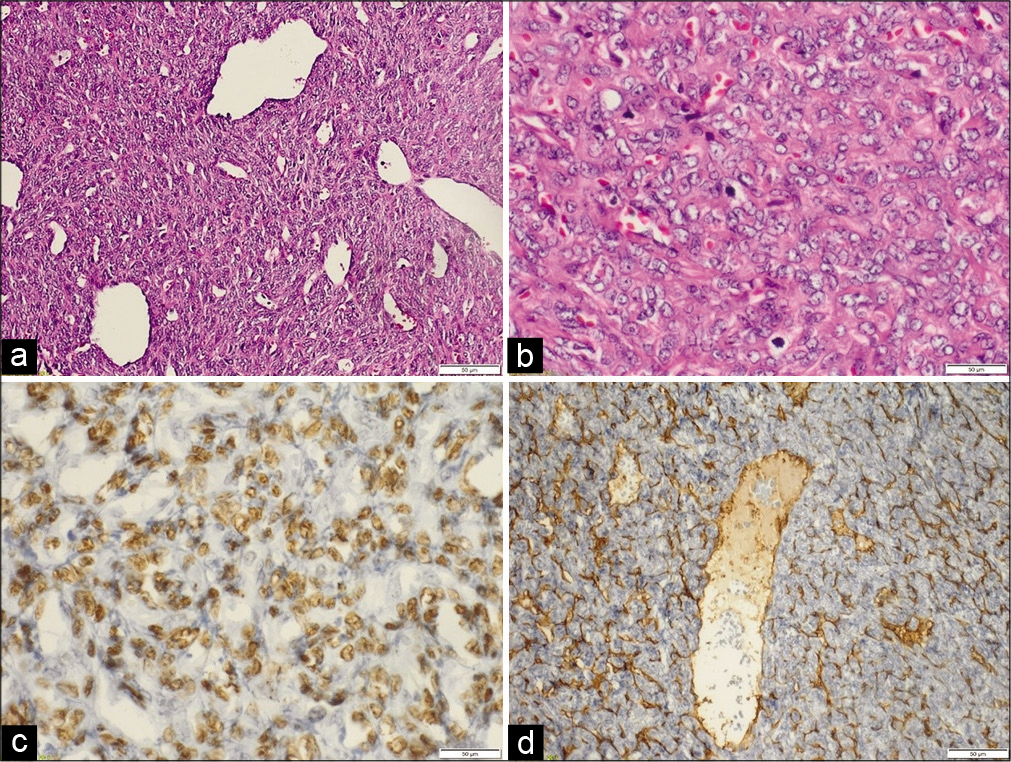
Figure 3:
Histopathological description. Malignant spindle cell proliferation, forming sheets of cells with a prominent staghorn (hemangiopericytoma) vascular pattern (a). The cells display plump spindles to ovoid monomorphic nuclei with open chromatin. Mitotic figures are noted (b). Tumor cells are diffusely positive for TLE 1 (c). CD34 and smooth muscle actin highlight the prominent vascular network (d).
DISCUSSION
Sarcomas are a group of heterogeneous tumors that predominantly arise from the embryonic mesoderm. One type of sarcoma is soft-tissue sarcoma (STS), which can occur nearly anywhere in the body, but most commonly in the extremities. A rare histological subtype of STS is SS, which accounts for 10% of all STS cases. Despite its name, it does not arise from the synovial membrane.[
Primary SS of the spine is particularly rare, with few case reports in the literature, and its etiology remains unclear. It can arise from the paraspinal muscles, paravertebral regions, or epidural spaces.[
Nerve sheath tumors such as neurofibroma, schwannoma, or malignant peripheral nerve sheath tumor (MPNST) are among the differential diagnosis in the presence of solid lesions.[
SS of the spine presents in several histopathological forms. Approximately 70% of all cases reported in the literature are monophasic, and the remaining 30% are either biphasic or poorly differentiated SS. Some cases, including the current case, exhibited characteristics of hemangiopericytoma patterns, and this may explain the high intraoperative bleeding.[
Surgical excision with negative margins of the tumor is considered the most effective treatment for SS; however, excision cannot be performed in most of the cases due to the important adjacent structures such as the spinal cord and spinal nerves. The use of adjuvant radiation and chemotherapy has been demonstrated to reduce local recurrence in several cases.[
There was no significance difference in the outcome among different treatment strategies. However, patients who underwent surgical excision alone experienced greater recurrence with distal metastases compared to other combined treatment strategies (most commonly lung metastases).[
CONCLUSION
Due to the limited number of reported cases in the literature, it is difficult to predict the outcomes of SS of the spine. Different treatment modalities have been used to treat spinal SS. However, most of the reported cases had poor outcomes. Therefore, prospective multi-center studies are needed to further investigate the treatment strategies and outcomes for patients with spinal SS.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Barus CE, Monsey RD, Kalof AN. Poorly differentiated synovial sarcoma of the lumbar spine in a fourteen-year-old girl. A case report. J Bone Joint Surg Am. 2009. 91: 1471-6
2. Chen Q, Shi F, Liu L, Song Y. Giant synovial sarcoma involved thoracolumbar vertebrae and paraspinal muscle. Spine J. 2016. 16: e271-2
3. Chu PG, Benhattar J, Weiss LM, Meagher-Villemure K. Intraneural synovial sarcoma: Two cases. Mod Pathol. 2004. 17: 258-63
4. De Ribaupierre S, Vernet O, Beck-Popovic M, Meagher-Villemure K, Rilliet B. Cervical nerve root synovial sarcoma in a child with chromosomal (X;18) translocation. Case report and review of the literature. Pediatr Neurosurg. 2007. 43: 382-5
5. Greene S, Hawkins DS, Rutledge JC, Tsuchiya KD, Douglas J, Ellenbogen RG. Pediatric intradural extramedullary synovial sarcoma: Case report. Neurosurgery. 2006. 59: E1339
6. Guo A, Guo F. Sudden onset of paraplegia secondary to an unusual presentation of pediatric synovial sarcoma. Childs Nerv Syst. 2016. 32: 2465-9
7. Kim J, Lee SH, Choi YL, Bae GE, Kim ES, Eoh W. Synovial sarcoma of the spine: A case involving paraspinal muscle with extensive calcification and the surgical consideration in treatment. Eur Spine J. 2014. 23: 27-31
8. Kim KW, Park SY, Won KY, Jin W, Kim SM, Park JS. Synovial sarcoma of primary bone origin arising from the cervical spine. Skeletal Radiol. 2013. 42: 303-8
9. Koehler SM, Beasley MB, Chin CS, Wittig JC, Hecht AC, Qureshi SA. Synovial sarcoma of the thoracic spine. Spine J. 2009. 9: e1-6
10. Liu ZJ, Zhang LJ, Zhao Q, Li QW, Wang EB, Ji SJ. Pediatric synovial sarcoma of the sacrum: A case report. J Pediatr Orthop B. 2010. 19: 207-10
11. Morrison C, Wakely PE, Ashman CJ, Lemley D, Theil K. Cystic synovial sarcoma. Ann Diagn Pathol. 2001. 5: 48-56
12. Mullah-Ali A, Ramsay JA, Bourgeois JM, Hodson I, MacDonald P, Midia M. Paraspinal synovial sarcoma as an unusual postradiation complication in pediatric abdominal neuroblastoma. J Pediatr Hematol Oncol. 2008. 30: 553-7
13. Najib S, Saleem T, Nadhim A, Sen S. A rare case of monophasic synovial sarcoma of thoracic vertebra. Case Rep Med. 2018. 2018: 2313927
14. Naphade PS, Desai MS, Shah RM, Raut AA. Synovial sarcoma of cervical intervertebral foramen: A rare cause of brachial weakness. Neurol India. 2011. 59: 783-5
15. Peia F, Gessi M, Collini P, Ferrari A, Erbetta A, Valentini LG. Pediatric primitive intraneural synovial sarcoma of L-5 nerve root. J Neurosurg Pediatr. 2013. 11: 473-7
16. Puffer RC, Daniels DJ, Giannini C, Pichelmann MA, Rose PS, Clarke MJ. Synovial sarcoma of the spine: A report of three cases and review of the literature. Surg Neurol Int. 2011. 2: 18
17. Ravnik J, Potrc S, Kavalar R, Ravnik M, Zakotnik B, Bunc G. Dumbbell synovial sarcoma of the thoracolumbar spine: A case report. Spine (Phila Pa 1976). 2009. 34: E363-6
18. Sakellaridis N, Mahera H, Pomonis S. Hemangiopericytoma-like synovial sarcoma of the lumbar spine. Case report. J Neurosurg Spine. 2006. 4: 179-82
19. Signorini GC, Pinna G, Freschini A, Bontempini L, Dalle Ore G. Synovial sarcoma of the thoracic spine. A case report. Spine (Phila Pa 1976). 1986. 11: 629-31
20. Subramanian S, Jonathan GE, Patel B, Prabhu K. Synovial sarcoma mimicking a thoracic dumbell schwannoma-a case report. Br J Neurosurg. 2020. 34: 98-101
21. Suh SI, Seol HY, Hong SJ, Kim JH, Kim JH, Lee JH. Spinal epidural synovial sarcoma: A case of homogeneous enhancing large paravertebral mass on MR imaging. AJNR Am J Neuroradiol. 2005. 26: 2402-5
22. Treu EB, De Slegte RG, Golding RP, Sperber M, Van Zanten TE, Valk J. CT findings in paravertebral synovial sarcoma. J Comput Assist Tomogr. 1986. 10: 460-2
23. Verbeke SL, Fletcher CD, Alberghini M, Daugaard S, Flanagan AM, Parratt T. A reappraisal of hemangiopericytoma of bone; analysis of cases reclassified as synovial sarcoma and solitary fibrous tumor of bone. Am J Surg Pathol. 2010. 34: 777-83
24. Yang C, Fang J, Xu Y. Primary cervical intramedullary synovial sarcoma: A longitudinal observation. Spine J. 2016. 16: e657-8
25. Yang M, Zhong N, Zhao C, Xu W, He S, Zhao J. Surgical management and outcome of synovial sarcoma in the spine. World J Surg Oncol. 2018. 16: 175
26. Yonezawa I, Saito T, Nakahara D, Won J, Wada T, Kaneko K. Synovial sarcoma of the cauda equina. J Neurosurg Spine. 2012. 16: 187-90
27. Zairi F, Assaker R, Bouras T, Chastanet P, Reyns N. Cervical synovial sarcoma necessitating multiple neurosurgical procedures. Br J Neurosurg. 2011. 25: 769-71