- Department of Neurosurgery, San Salvatore city Hospital, L’Aquila, Italy
- Department of Pathology, San Salvatore city Hospital, L’Aquila, Italy
- Department of Life, Health & Environmental Sciences (MESVA), University of L’Aquila, Italy
Correspondence Address:
Danilo De Paulis
Department of Life, Health & Environmental Sciences (MESVA), University of L’Aquila, Italy
DOI:10.4103/sni.sni_401_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Alessandro Ricci, Hambra Di Vitantonio, Danilo De Paulis, Mattia Del Maestro, Soheila Raysi Dehcordi, Domenico Murrone, Gino Coletti, Giuseppe Calvisi, Renato Juan Galzio. Systemic sclerosis associated with colliquative necrosis in the cerebellum. 05-Apr-2017;8:44
How to cite this URL: Alessandro Ricci, Hambra Di Vitantonio, Danilo De Paulis, Mattia Del Maestro, Soheila Raysi Dehcordi, Domenico Murrone, Gino Coletti, Giuseppe Calvisi, Renato Juan Galzio. Systemic sclerosis associated with colliquative necrosis in the cerebellum. 05-Apr-2017;8:44. Available from: http://surgicalneurologyint.com/surgicalint-articles/systemic-sclerosis-associated-with-colliquative-necrosis-in-the-cerebellum/
Abstract
Background:The scleroderma is a complex autoimmune collagen disorder that can affect many organs simultaneously, as it occurs in the systemic sclerosis (SS), or only the skin, as it occurs in the localized scleroderma (LS). The neurological presentation is extremely uncommon, and even more uncommon are the symptoms of the scleroderma in the cerebellum.
Case Description:We report the case of a 56-year-old male with cerebellar lesions mimicking a brain abscess. After surgical excision, the histopathological diagnosis deposed for an ischemic necrosis caused by a vasculopathy. All the bacteriological and viral exams were negative, whereas the rheumatologic tests were compatible with the scleroderma pattern.
Conclusion:Up to now, the literature has described only 5 cases of scleroderma in the posterior cranial fossa. The authors report a case of SS causing colliquative necrosis in the cerebellum. Pathogenetic mechanisms, clinical aspects, and radiological features are discussed along with the pertinent literature.
Keywords: Cerebellum, colliquative necrosis, posterior cranial fossa, scleroderma
INTRODUCTION
The term “scleroderma” literally means “hard skin,”[
In general, the neurological presentation is extremely rare.[
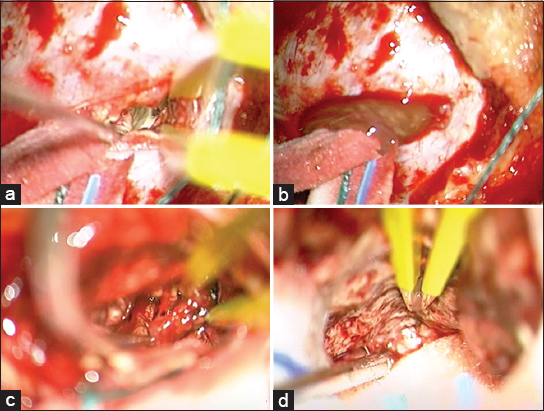
The authors report the first case of ischemic necrosis of the cerebellum in a patient with SS mimicking a brain abscess. Clinical aspects, radiological features, surgical treatment, and operative findings are discussed along with a review of the pertinent literature.
CASE REPORT
A 56-year-old male was admitted to our Institute with a sudden headache and dizziness accompanied by nausea and vomiting. The patient had been in good health until this event, except for a history of skin thickening, a Raynaud's phenomenon with acrocyanosis to the fingers from 1 year and arthralgia from 3 years. Other sclerotic lesions were observed on the knees and elbows.
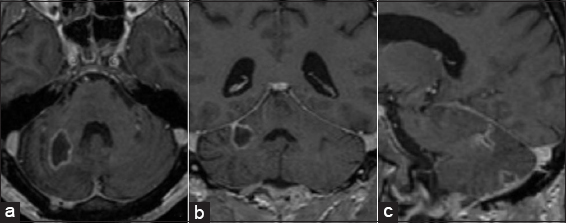
On admission, his blood pressure, pulse, body temperature, and respiration were normal. The neurological examination showed dysmetria, nystagmus, and dizziness. There were neither sensory nor motor deficits and cranial nerves abnormality. The results of the routine hematologic tests showed only neutrophilic leukocytosis with relative lymphocytopenia. The initial brain computerized tomography (CT) scan and the subsequent magnetic resonance imaging (MRI) showed a lesion with fluid collection, measuring 1.5 × 3.5 cm in the right cerebellar hemisphere [
Figure 3
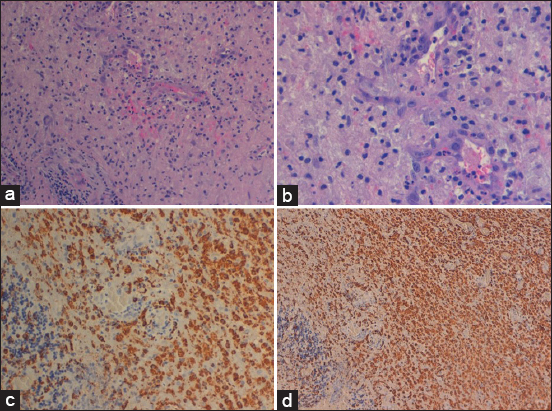
Imaging in hematoxylin and eosin and original magnification ×20 and ×40 showed cerebellar necrosis associated with microparenchymal hemorrhage, leukocytoclastic vasculitis, and swollen endothelial cells (a and b). Immunohistochemistry imaging (CD 68 +) showed massive macrophages infiltration in the cerebellar parenchyma (c and d)
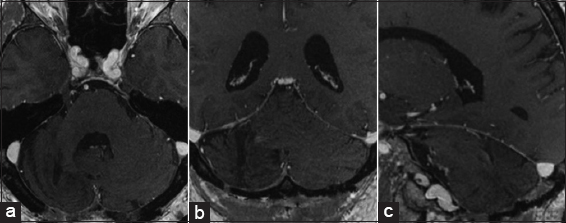
Further investigations tested positive for antinuclear antibody (ANA) >1:160 and Scl-70 (16.3 UA/mL), whereas other tests for rheumatology and infectious diseases tested negative (antiphospholipid anticentromere and anticardiolipin antibodies, Lupus-like anticoagulant, anti-DNA autoantibodies, perinuclear antineutrophil antibodies (P-ANCA), cytoplasmic antineutrophil antibodies (C-ANCA), Anti-Smith antibodies (Anti-Sm), Anti-Sjögren’s-syndrome-related antigen A/B (Anti-SSA/B), Antibodies against antigen Ro52 (Anti-Ro52), antibodies histidyl-tRNA synthetase Jo-1 (Anti-Jo1), major centromere autoantigen B (CENP-B), Nucleosomes, Ribosomal P- Protein, Anti-Ribonuclear Protein (Anti-U1RNP), Histones, Protein S Factor V Leiden, Hepatitis B surface antigen (HBs-Ag), Hepatitis C Core antibodies (HCV-Ab), antibodies Human Immunodeficiency Virus (HIV-Ab), Veneral Disease Research Laboratory). In addition to this, it was found an increase of circulating immune complexes (38 μg/ml), α1-acid-glycoprotein (208 mg/dl) and C-reactive protein (0.80 mg/dl). The capillaroscopy in the periungual area showed slight disorganization of the capillary architecture, numerous giant capillaries and microhemorrhages, moderate capillary loss, and few ramified capillaries with initial neoangiogenesis. The skin biopsy confirmed a focal extension of dermal collagen in the subcutaneous tissue. All these conditions were compatible with a scleroderma pattern. The patient was sent to our rheumatology reference center to start a specific therapy. At 1-year follow-up there were no other complications.
DISCUSSION
In general, the scleroderma represents a complex autoimmune collagen disorder.[
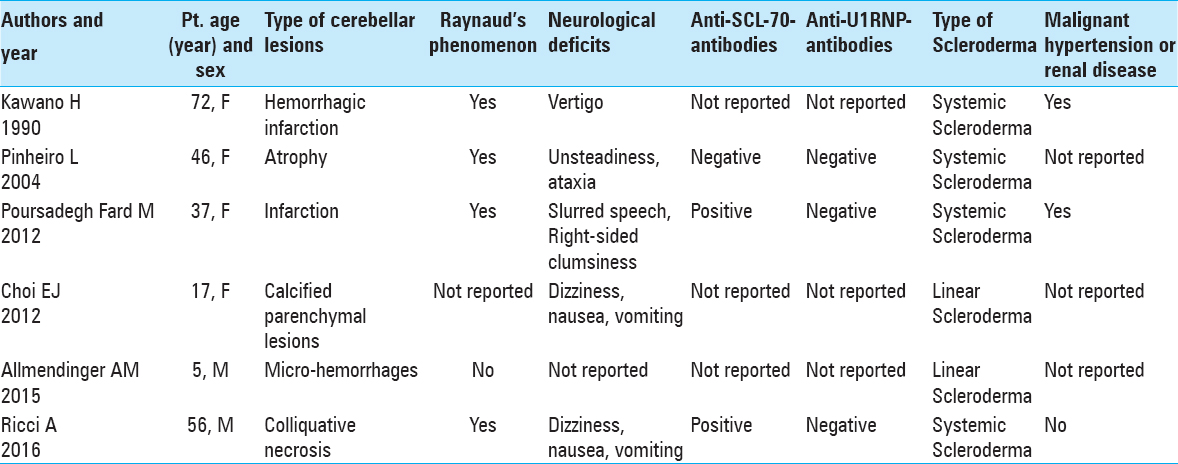
Currently, only 5 cases have been described in literature.[
In literature, no studies have correlated the posterior circulation and cerebellum with SS, whereas several theories have been proposed to explain the mechanism of a vascular damage caused by the SS in CNS. Some authors think that the pathogenic mechanism is caused both by the cell-mediated activity and autoantibody production responsible for a chronic inflammation. This determines a progressive fibrosis of the visceral organs and vascular damage, including the intracranial vessels.[
Other studies have shown that the vasculopathy in the SS is caused by an obliterative vasculopathy rather than by the classical atherosclerosis.[
In general, the cerebral angiopathy in the scleroderma is associated with the presence of malignant hypertension or renal disease,[
Other rare CNS abnormalities described include encephalopathy, subarachnoid hemorrhage, psychosis, anxiety, and trigeminal neuropathy.[
There are few reports on the possible associations of different autoantibodies with neurological manifestations of scleroderma.[
We think that the involvement of the CNS is unpredictable, and according to Mohammed et al.,[
CONCLUSION
We believe that in our case the cerebellar lesion was caused by microangiophaty of the posterior circulation secondary to a high activity of the SS. The pathogenetic mechanism that causes the colliquative necrosis might be generated by microglia and macrophages surrounding the cerebellar vessels. In response to inflammatory stimuli, secondary to the scleroderma effect on the microcirculation, the microglia rapidly transform into an activated phenotype, elaborating both neurotoxic and neurotrophic factors responsible for the formation of a colliquative collection.
In our case, the first manifestation of the scleroderma is cerebellar, and therefore, in the presence of a brain lesion similar to an abscess, it is recommended to include autoimmune diseases such as the scleroderma among the diagnostic hypotheses.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors thank Maria Silvia Marottoli for her assistance in the translation.
References
1. Allmendinger AM, Ricci JA, Desai NS, Viswanadhan N, Rodriguez D. Atypical Neuroimaging Manifestations of Linear Scleroderma “en coup de sabre”. Iran J Child Neurol. 2015. 9: 62-8
2. Chiang CH, Liu CJ, Huang CC, Chan WL, Huang PH, Chen TJ. Systemic sclerosis and risk of ischaemic stroke: A nationwide cohort study. Rheumatology. 2013. 52: 161-5
3. Choi EJ, Lee DW, Park CW, Lee SH. A case of linear scleroderma involving cerebellum with vertigo. Korean J Audiol. 2012. 16: 87-90
4. Das CP, Prabhakar S, Lal V, Kharbanda PS. Scleroderma, stroke, optic neuropathy: A rare association. Neurol India. 2002. 50: 504-7
5. Estey E, Lieberman A, Pinto R, Meltzer M, Ransohoff J. Cerebral arteritis in scleroderma. Stroke. 1979. 10: 595-7
6. Héron E, Fornes P, Rance A, Emmerich J, Bayle O, Fiessinger JN. Brain involvement in scleroderma: Two autopsy cases. Stroke. 1998. 29: 719-21
7. Hietarinta M, Lassila O, Hietaharju A. Association of anti-U1RNP- and anti-Scl-70-antibodies with neurological manifestations in systemic sclerosis (scleroderma). Scand J Rheumatol. 1994. 23: 64-7
8. Kanzato N, Matsuzaki T, Komine Y, Saito M, Saito A, Yoshio T, Suehara M. Localized scleroderma associated with progressing ischemic stroke. Neurol Sci. 1999. 163: 86-9
9. Kawano H, Hayashi M, Handa Y, Miyazaki S. A case of progressive systemic sclerosis associated with a hemorrhagic infarction of the cerebellum. No To Shinkei. 1990. 42: 189-91
10. Mohamed RH, Nassef AA. Brain magnetic resonance imaging findings in patients with systemic sclerosis. Int J Rheum Dis. 2010. 13: 61-7
11. Mohammed RH, Sabry YY, Nasef AA. Brain MRI screening showing evidences of early central nervous system involvement in patients with systemic sclerosis. Rheumatol Int. 2011. 31: 667-71
12. Pathak R, Gabor AJ. Scleroderma and central nervous system vasculitis. Stroke. 1991. 22: 410-3
13. Pinheiro L, Freitas J, Lucas M, Victorino RM. Cerebellar atrophy in systemic sclerosis. J R Soc Med. 2004. 97: 537-8
14. Prescott RJ, Freemont AJ, Jones CJ, Hoyland J, Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol. 1992. 166: 255-6
15. Poursadegh Fard M, Karami Magham S. Cerebral sinus thrombosis in scleroderma: A case report. Acta Med Iran. 2012. 50: 288-91
16. Roquer J, Segura T, Serena J, Castillo J. Endothelial dysfunction, vascular disease and stroke: The ARTICO study. Cerebrovasc Dis. 2009. 27: 25-37
17. Soltész P, Kerekes G, Dér H, Szücs G, Szántó S, Kiss E. Comparative assessment of vascular function in autoimmune rheumatic diseases: Considerations of prevention and treatment. Autoimmun Rev. 2011. 10: 416-25
18. Tuffanelli DL, Winkelmann RK. Systemic scleroderma, a clinical study of 727 cases. Arch Dermatol. 1961. 84: 359-71