- Neurosurgery, Department of Surgical Specialties, Pedro Ernesto University Hospital, Rio de Janeiro, Brazil
- Department of Pathology, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brazil.
Correspondence Address:
Maria Eduarda Rosário Viveiros de Castro, Neurosurgery, Department of Surgical Specialties, Pedro Ernesto University Hospital, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brazil.
DOI:10.25259/SNI_1017_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Maria Eduarda Rosário Viveiros de Castro1, Pedro Henrique Costa Ferreira-Pinto1, Domênica Baroni Coelho de Oliveira Ferreira1, Ana Carolina Gonçalves Brito2, Maud Parise1, Eduardo Mendes Correa1, Thaina Zanon Cruz1, Wesley Klein Nunes de Freitas1, Pedro Luiz Ribeiro Carvalho de Gouvea1, Wellerson Novaes da Silva1, Bruna Cavalcante de Sousa1, Hannah Ferreira Machado Videira1, Guilherme Freitas Parra1, Flavio Nigri1. Temporal bone squamous cell carcinoma: Aggressive behavior coursing with cerebellar invasion and hydrocephalus. 15-Mar-2024;15:89
How to cite this URL: Maria Eduarda Rosário Viveiros de Castro1, Pedro Henrique Costa Ferreira-Pinto1, Domênica Baroni Coelho de Oliveira Ferreira1, Ana Carolina Gonçalves Brito2, Maud Parise1, Eduardo Mendes Correa1, Thaina Zanon Cruz1, Wesley Klein Nunes de Freitas1, Pedro Luiz Ribeiro Carvalho de Gouvea1, Wellerson Novaes da Silva1, Bruna Cavalcante de Sousa1, Hannah Ferreira Machado Videira1, Guilherme Freitas Parra1, Flavio Nigri1. Temporal bone squamous cell carcinoma: Aggressive behavior coursing with cerebellar invasion and hydrocephalus. 15-Mar-2024;15:89. Available from: https://surgicalneurologyint.com/surgicalint-articles/12800/
Abstract
Background: Temporal bone squamous cell carcinoma (TBSCC) is a very rare condition. The prognosis is dismal for advanced tumors. Due to its rarity, information in the literature is scarce. Here, we report a unique case of TBSCC with cerebellar invasion and hydrocephalus.
Case Description: A 46-year-old reported right-sided hearing loss and a painful right retroauricular mass for 4 months. Magnetic resonance imaging revealed a 8.7 × 7.6 × 6.4 cm mass invading the right temporal and occipital bones. After a biopsy and 3 surgical procedures over 6 months, the diagnosis of TBSCC was obtained. Due to invasion of the cerebellar tissue and obstructive hydrocephalus, a ventriculoperitoneal shunt was performed. The patient was referred for adjuvant radiotherapy. However, palliative care was initiated due to tumor progression.
Conclusion: We report a case of advanced TBSCC with poor prognosis despite surgical treatment and radiotherapy. More data are necessary to provide new and better treatment to these patients.
Keywords: Cerebellum, Hydrocephalus, Squamous cell carcinoma, Temporal bone
INTRODUCTION
Temporal bone squamous cell carcinoma (TBSCC) is a rare head and neck tumor with aggressive behavior.[
CASE DESCRIPTION
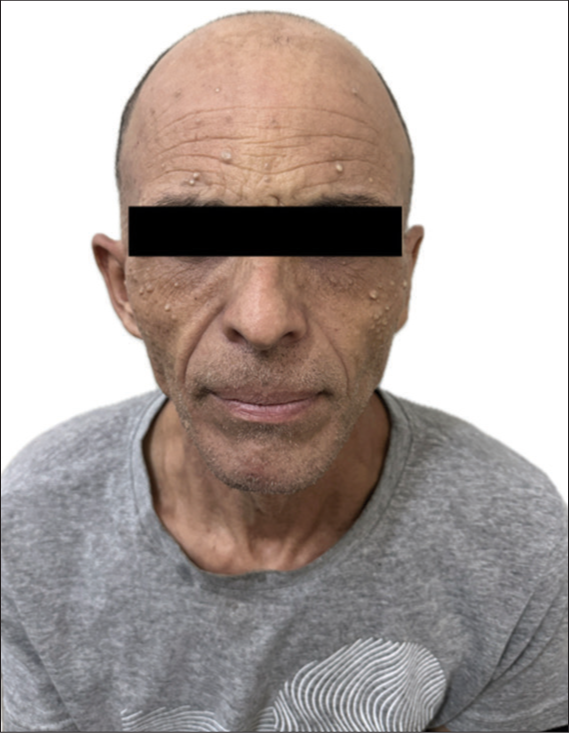
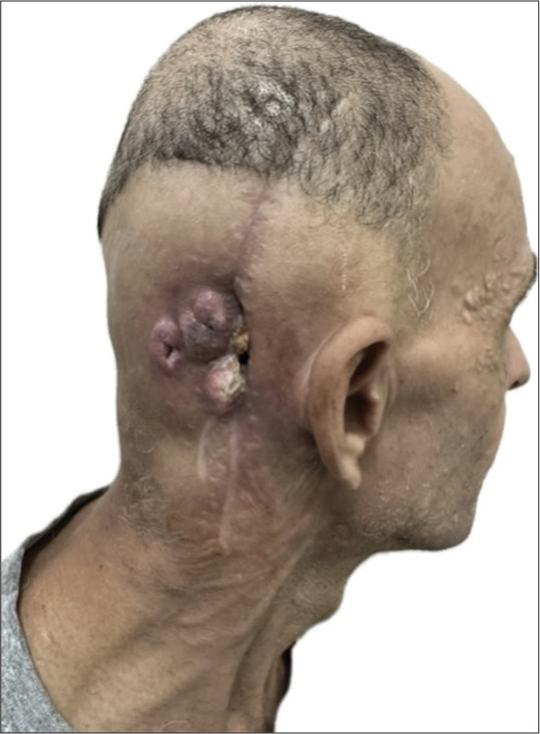
A 46-year-old longshoreman went to a University Hospital reporting right-sided hearing loss and a painful right retroauricular mass, which was noticed 4 months earlier after being unable to wear his safety helmet. He also reported rapid weight loss of 4 kg in 1 month. His only previous comorbidity was bilateral occupational hypoacusis. Clinical examination revealed a right retroauricular mass, in addition to right anacusis and left hypoacusis. On careful inspection, there was no evidence of skin lesions other than diffuse milia [
Figure 2:
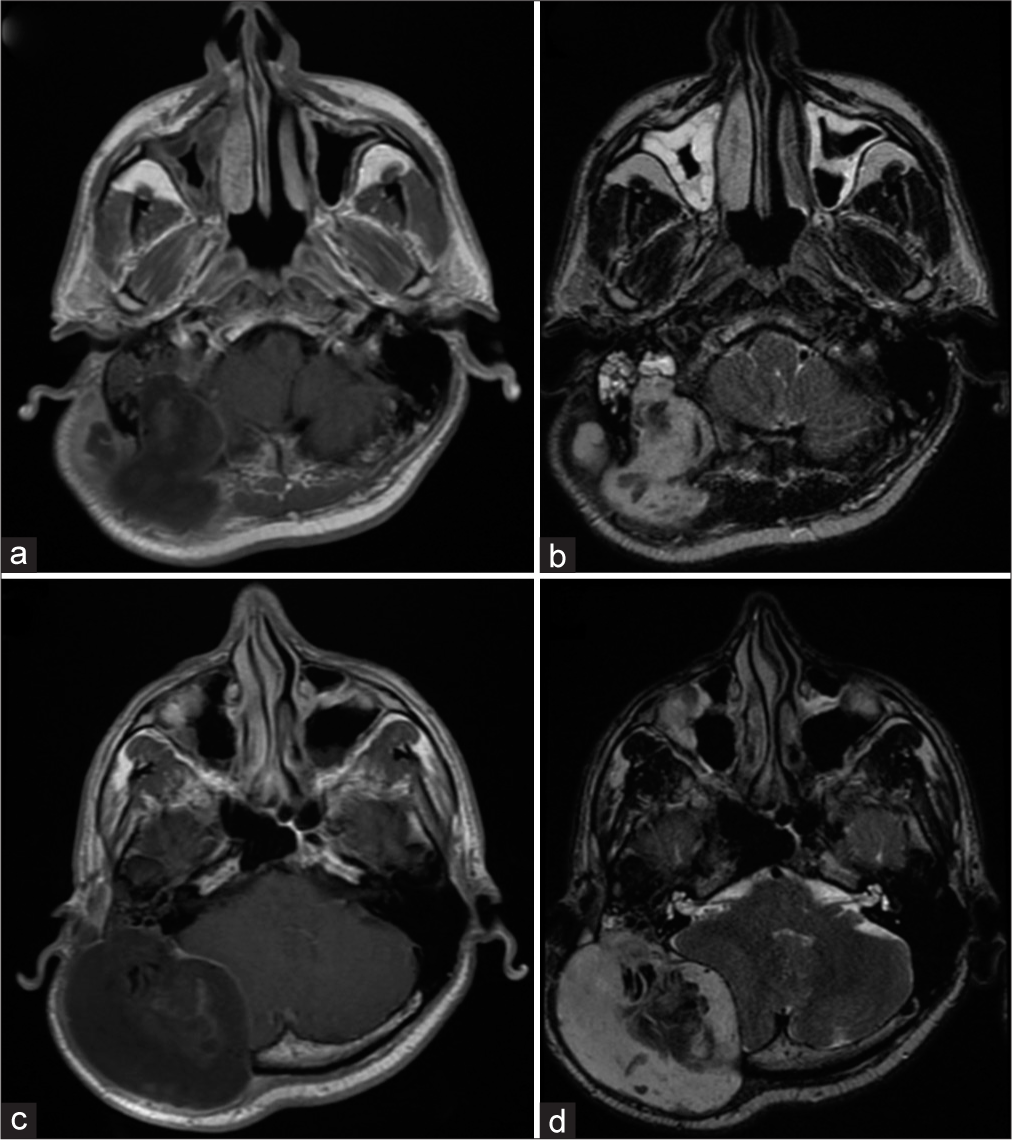
First magnetic resonance imaging of the brain showing a huge lesion in the temporal bone. (a) Axial T1-weighted with contrast exhibiting temporal bone mass with peripheral contrast enhancement – right side. (b) Axial T2-weighted image shows hyperintense signal abnormality in the mastoid part of the temporal bone. The occipital bone was invaded. (c) Contrast-enhanced axial T1-weighted showing a large temporal bone tumor on the right side. (d) The mass was predominantly hyperintense on the T2-weighted image.
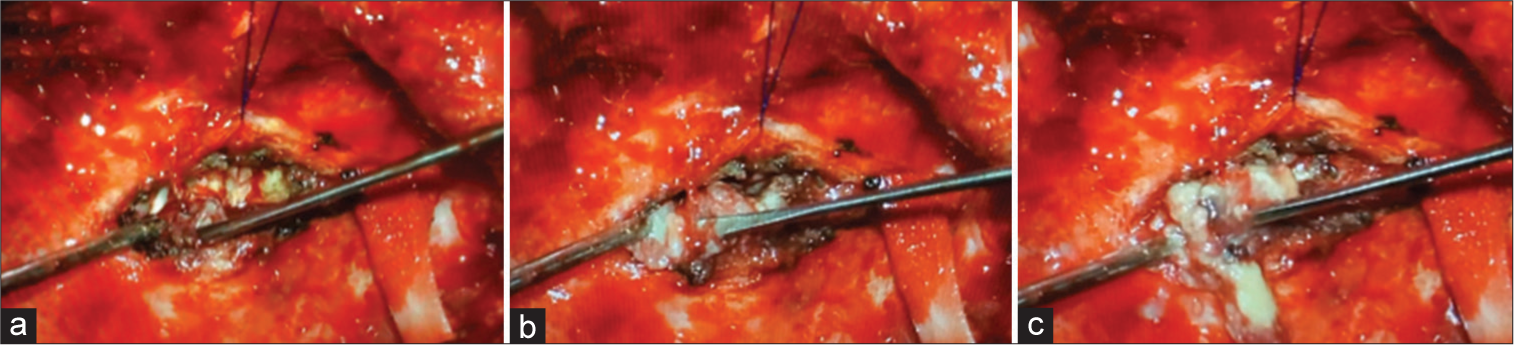
A tumor biopsy was performed and the histopathological findings were negative for malignancy and infection screening (GeneXpert – tuberculosis, Gram staining, and mycological culture). After that, due to the rapid tumor growth, he underwent another 2 surgical partial resections with positive margins over a period of 5 months. The tumor was not completely resected due to invasion of petrous bone and the risk of injury to the facial nerve. In the first surgical approaches, samples from the temporal bone tumor were predominantly represented by keratin sheets with parakeratosis, sometimes forming horny pearls, with extensive degenerated areas. Foci of preserved squamous epithelium were observed, showing areas with enlarged nuclei, but without signs of stromal invasion and criteria for malignancy. The sample was also subjected to an immunohistochemical study with p16, which was negative.
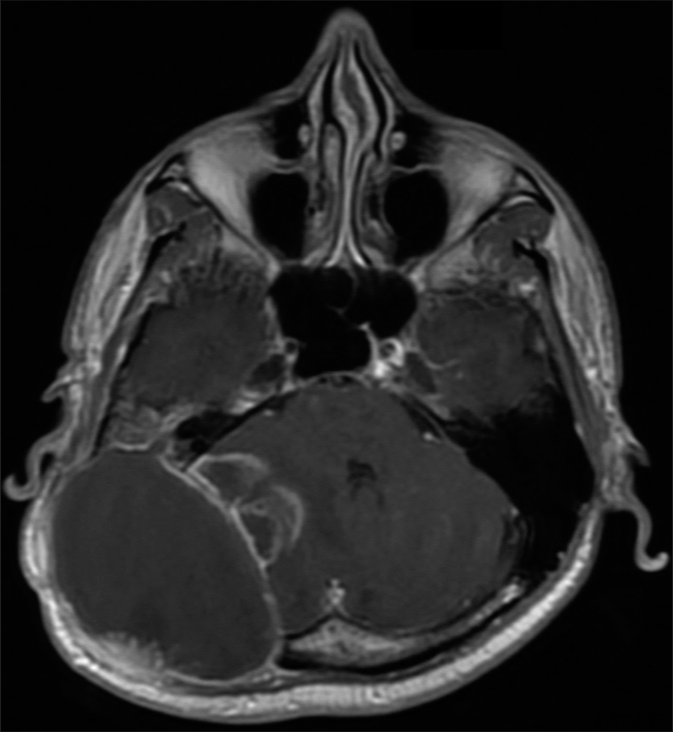
Two months after the last surgery, a new brain MRI revealed local recurrence and invasion of the cerebellar tissue [
One month after the last procedure, the patient exhibited a recurrent pattern of bulging deformity with a new holocranial headache. He did not present focal deficit symptoms. The new MRI revealed infiltration of the mass into the cerebellar region, accompanied by compression of the fourth ventricle and obstructive hydrocephalus [
Figure 5:
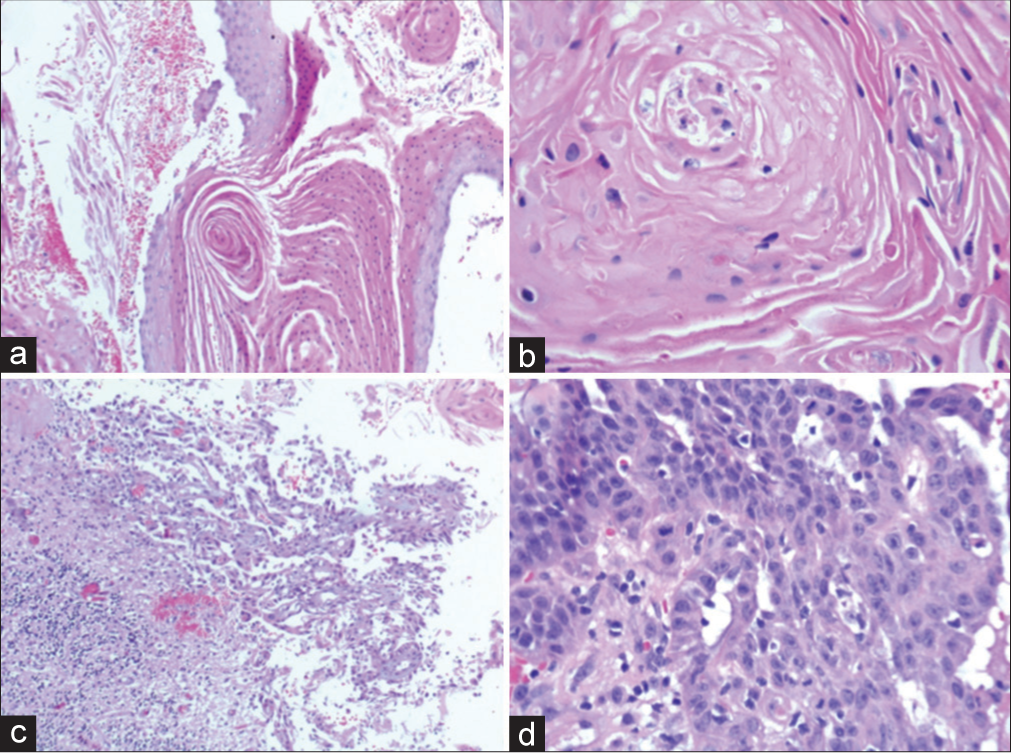
Temporal bone tumor samples: (a) Hematoxylin and Eosin (H&E) stain (×200). Squamous epithelium with some maturation, keratin sheets with parakeratosis, sometimes forming horny pearls. (b) H&E stain (×400). Foci of preserved squamous epithelium showing areas with enlarged nuclei, but no signs of stromal invasion. (c) H&E stain (×100). Sample from the cerebellar region. Squamous epithelium presenting poorly cohesive cells with moderate to marked pleomorphism, superficially infiltrating the cerebellar cortex. (d) H&E stain (×400). Sample from the dural region. Squamous epithelium with an enlarged and irregular nucleus, and frequent atypical mitotic figures infiltrating fibroconnective tissue.
Figure 6:
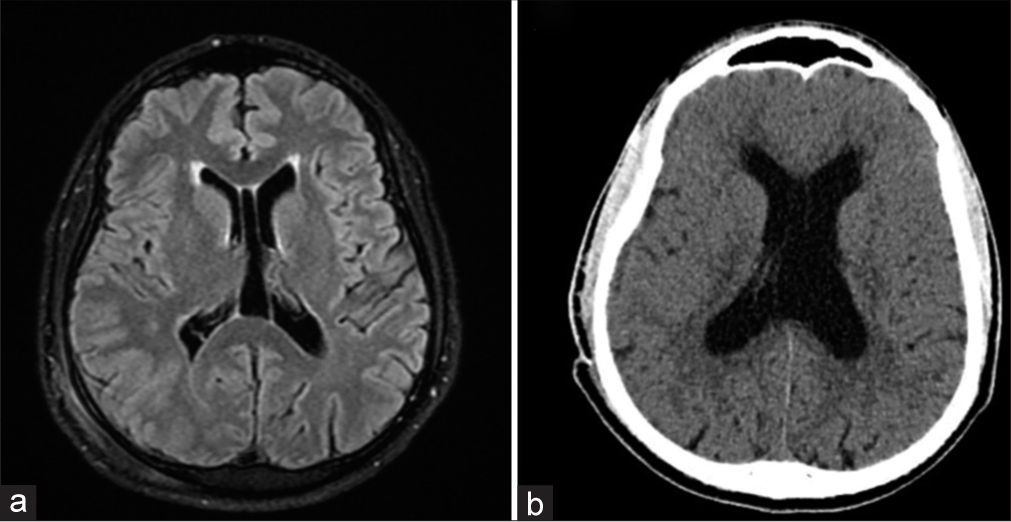
Comparison between the first brain magnetic resonance imaging (MRI) (fluid-attenuated inversion recovery [FLAIR] sequence) and head computed tomography (CT) after symptoms of hydrocephalus. (a) Preoperative MRI-FLAIR shows a normal lateral ventricle without radiological signs of hydrocephalus. Normal anatomical variation is observed: Cavum septum pellucidum. (b) Head CT showing enlargement of the lateral ventricles and transependymal edema: Low-density change on CT around the margins of the ventricles.
DISCUSSION
Temporal bone malignancies are rare, constituting only 0.2% of all head and neck malignancies. The global annual incidence is estimated to be 1.3 cases per million, and it is detected in approximately one in every 5000–20000 patients presenting with otological complaints.[
In contrast to other head and neck cancers, the risk of primary TBSCC does not appear to be significantly elevated by tobacco and alcohol consumption. Instead, prior radiation emerges as a notable risk factor. In addition, chronic otitis media, otitis externa, and cholesteatoma have been identified as potential contributors to primary TBSCC.[
The principal clinical indicators of temporal bone malignancies include otalgia, otorrhea, and hearing loss. Gradually, signs indicating more serious conditions, such as facial weakness or the presence of a parotid or neck mass, may manifest.[
Given that a substantial portion of the temporal bone is typically not directly visible, imaging studies play a crucial role in both staging and management. CT scan and MRI provide complementary information about the extent of the tumor.[
The main staging system used for temporal bone malignancies is the Pittsburgh Staging System (PSS). The PSS adopts the familiar tumor-node-metastasis (TNM) format, relying on CT findings such as bone external auditory canal (EAC) destruction, surrounding soft-tissue infiltration, and involvement of medial bony temporal structures. This approach allows the categorization of patients into treatment and prognostic groups. TNM can be transformed into the standard four-stage system used for other head and neck cancers, with the exception that any temporal bone malignancy with lymph node involvement is automatically classified as stage IV.[
Due to the rare occurrence of TBSCC, there is a paucity of adequate randomized trials, leading to wide variation in the extent of resection among different authors and institutions. In general, indicators of low disease-specific survival rates in patients with TBSCC include nodal involvement, poorly differentiated histology, carotid involvement, positive margins, stage T4, dural invasion, and temporomandibular joint invasion.[
Regarding surgical treatment, we considered the significant morbidity associated with total resection of the temporal bone and the absence of proven survival benefit.[
Radiotherapy for TBSCC is typically administered in the adjuvant postoperative period. Some indications include lymph node metastasis, perineural invasion, positive margins, recurrent tumors, and bone invasion.[
CONCLUSION
TBSCC is a very aggressive tumor associated with poor prognosis. We reported a unique case coursing with cerebellar invasion and hydrocephalus despite multiple surgical resections. There was also tumor progression after radiotherapy. More studies on this topic are necessary to provide new and better treatment to these patients.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Center of High Complexity Neurosurgery Intern Patients (NIPNAC).
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Allanson BM, Low TH, Clark JR, Gupta R. Squamous cell carcinoma of the external auditory canal and temporal bone: An update. Head Neck Pathol. 2018. 12: 407-18
2. Arena S, Keen M. Carcinoma of the middle ear and temporal bone. Am J Otol. 1998. 9: 351-6
3. Arriaga M, Curtin H, Takahashi H, Hirsch BE, Kamerer DB. Staging proposal for external auditory meatus carcinoma based on preoperative clinical examination and computed tomography findings. Ann Otol Rhinol Laryngol. 1990. 99: 714-21
4. Conley JJ. Cancer of the middle ear and temporal bone. N Y State J Med. 1974. 74: 1575-9
5. Gidley PW, DeMonte F. Temporal bone malignancies. Neurosurg Clin N Am. 2013. 24: 97-110
6. Kunst H, Lavieille JP, Marres H. Squamous cell carcinoma of the temporal bone: Results and management. Otol Neurotol. 2008. 29: 549-52
7. Leonetti JP, Smith PG, Kletzker GR, Izquierdo R. Invasion patterns of advanced temporal bone malignancies. Am J Otol. 2000. 122: 882-6
8. Lovin BD, Gidley PW. Squamous cell carcinoma of the temporal bone: A current review. Laryngoscope Investig Otolaryngol. 2019. 4: 684-92
9. Madsen AR, Gundgaard MG, Hoff CM, Maare C, Holmboe P, Knap M. Cancer of the external auditory canal and middle ear in Denmark from 1992 to 2001. Head Neck. 2008. 30: 1332-8
10. Masterson L, Winder D, Marker A, Sterling JC, Sudhoff HH, Moffat DA. Investigating the role of human papillomavirus in squamous cell carcinoma of the temporal bone. Head Neck Oncol. 2013. 5: 22-9
11. Mazzoni A, Danesi G, Zanoletti E. Primary squamous cell carcinoma of the external auditory canal: Surgical treatment and long-term outcomes. Acta Otorhinolaryngol Ital. 2014. 34: 129-37
12. McRackan TR, Fang TY, Pelosi S, Rivas A, Dietrich MS, Wanna GB. Factors associated with recurrence of squamous cell carcinoma involving the temporal bone. Ann Otol Rhinol Laryngol. 2014. 123: 235-9
13. Moffat DA, Wagstaff SA. Squamous cell carcinoma of the temporal bone. Curr Opin Otolaryngol Head Neck Surg. 2003. 11: 107-11
14. Moody SA, Hirsch BE, Myers EN. Squamous cell carcinoma of the external auditory canal: An evaluation of a staging system. Am J Otol. 2000. 21: 582-8
15. Ngu CY, Mohd Saad MS, Tang IP. Temporal bone squamous cell carcinoma: A change in treatment. Med J Malaysia. 2021. 76: 725-30
16. Prasad S, Janecka IP. Efficacy of surgical treatment for squamous cell carcinoma of the temporal bone: A literature review. Otolaryngol Head Neck Surg. 1994. 110: 270-80
17. Yin M, Ishikawa K, Honda K, Arakawa T, Harabuchi Y, Nagabashi T. Analysis of 95 cases of squamous cell carcinoma of the external and middle ear. Auris Nasus Larynx. 2006. 33: 251-7
18. Zanoletti E, Marioni G, Stritoni P, Lionello M, Giacomelli L, Martini A. Temporal bone squamous cell carcinoma: Analyzing prognosis with univariate and multivariate models. Laryngoscope. 2014. 124: 1192-8