- Department of Neurosurgery, Hospital Santa Casa de Belo Horizonte, Belo Horizonte, Minas Gerais, Brazil
- Faculty of Medical Sciences of Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
- Department of Neurosurgery, Hospital das Clínicas de Belo Horizonte, Belo Horizonte, Minas Gerais, Brazil
Correspondence Address:
Warley Carvalho da Silva Martins
Department of Neurosurgery, Hospital Santa Casa de Belo Horizonte, Belo Horizonte, Minas Gerais, Brazil
DOI:10.4103/sni.sni_95_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Warley Carvalho da Silva Martins, Lucas Alverne Freitas de Albuquerque, Gervásio Teles Cardoso de Carvalho, Jules Carlos Dourado, Marcos Dellaretti, Atos Alves de Sousa. Tenth case of bilateral hemifacial spasm treated by microvascular decompression: Review of the pathophysiology. 26-Sep-2017;8:225
How to cite this URL: Warley Carvalho da Silva Martins, Lucas Alverne Freitas de Albuquerque, Gervásio Teles Cardoso de Carvalho, Jules Carlos Dourado, Marcos Dellaretti, Atos Alves de Sousa. Tenth case of bilateral hemifacial spasm treated by microvascular decompression: Review of the pathophysiology. 26-Sep-2017;8:225. Available from: http://surgicalneurologyint.com/surgicalint-articles/tenth-case-of-bilateral-hemifacial-spasm-treated-by-microvascular-decompression-review-of-the-pathophysiology/
Abstract
Background:Bilateral hemifacial spasm (BHFS) is a rare neurological syndrome whose diagnosis depends on excluding other facial dyskinesias. We present a case of BHFS along with a literature review.
Methods:A 64-year-old white, hypertense male reported involuntary left hemiface contractions in 2001 (aged 50). In 2007, right hemifacial symptoms appeared, without spasm remission during sleep. Botulinum toxin type A application produced partial temporary improvement. Left microvascular decompression (MVD) was performed in August 2013, followed by right MVD in May 2014, with excellent results. Follow-up in March 2016 showed complete cessation of spasms without medication.
Results:The literature confirms nine BHFS cases bilaterally treated by MVD, a definitive surgical option with minimal complications. Regarding HFS pathophysiology, ectopic firing and ephaptic transmissions originate in the root exit zone (REZ) of the facial nerve, due to neurovascular compression (NVC), orthodromically stimulate facial muscles and antidromically stimulate the facial nerve nucleus; this hyperexcitation continuously stimulates the facial muscles. These activated muscles can trigger somatosensory afferent skin nerve impulses and neuromuscular spindles from the trigeminal nerve, which, after transiting the Gasser ganglion and trigeminal nucleus, reach the somatosensory medial posterior ventral nucleus of the contralateral thalamus as well as the somatosensory cortical area of the face. Once activated, this area can stimulate the motor and supplementary motor areas (extrapyramidal and basal ganglia system), activating the motoneurons of the facial nerve nucleus and peripherally stimulating the facial muscles.
Conclusions:We believe that bilateral MVD is the best approach in cases of BHFS.
Keywords: Bilateral hemifacial spasm, botulinum toxin, microvascular decompression
INTRODUCTION
Hemifacial spasm (HFS) is a common, involuntary, intermittent movement disorder induced in most cases by neurovascular compression (NVC) in the root exit zone (REZ) of the ipsilateral facial nerve in the brainstem.[
To our knowledge, this is the tenth case of bilateral HFS (BHFS) treated by microvascular decompression (MVD) to be described. A comprehensive review of the literature follows the case report.
MATERIALS AND METHODS/CASE MATERIAL
Case report
A 64-year-old, white, hypertense male reported the onset of involuntary contractions of the left orbicularis muscle in 2001 (aged 50), which progressed to contractions of all the muscles in this hemiface. In 2007, symptoms also appeared in the right orbicularis muscle and again progressed to all muscles in this hemiface. The patient reported no remission of spasms in either hemiface during sleep. Carbamazepine was prescribed (200 mg BID) with slight improvement in the symptoms initially, followed by relapses. Next, he was submitted to applications of botulinum toxin type A (BTX-A), which produced partial temporary improvement.
The course of the disease included bilateral progressive hearing loss. In September 2012, he was submitted to audiometry, which showed mild to moderate sensorineural loss (descending audiometric curve) in both ears. Magnetic resonance imaging (MRI) was performed in October 2012 and showed that the left vertebral artery followed a tortuous and elongated path, compressing and dislocating ipsilateral cranial nerves VII and VIII, compatible with neurovascular conflict, and discrete tortuosity of the right posterior inferior cerebellar artery (PICA), touching the brainstem along the REZ of the VII-VIII nerve complex on the right side.
The patient was submitted to left MVD in August 2013 because the symptoms were more pronounced in this hemiface. He evolved with complete cessation of spasms in the left hemiface, presenting moderate postoperative peripheral facial paresis with progressive and full improvement in December, though the left hypoacusis remained. In May 2014, he was submitted to right MVD, again presenting moderate postoperative peripheral facial paresis, with full recovery in 3 weeks. All right HFS ceased, but the previous right hypoacusis remained.
He remained free from facial spasms until August 2014, when he began to experience mild relapse in the right hemiface, with two to three fleeting spasms of the orbicularis muscle per hour and no episodes during sleep, which had previously been an important complaint. His health status remained the same during 19 months of follow-up. In March 2016, he returned for evaluation showing cessation of spasms without medication. The hearing deficits did not improve.
RESULTS AND DISCUSSION
Epidemiology
The annual incidence of HFS is 0.81 per 100,000 in women and 0.74 per 100,000 in men. The average prevalence is 11 per 100,000 in the general population. Women develop the disease in 14.5 per 100,000 individuals and men in 7.4 per 100,000 individuals, thus female:male distribution is 2:1.[
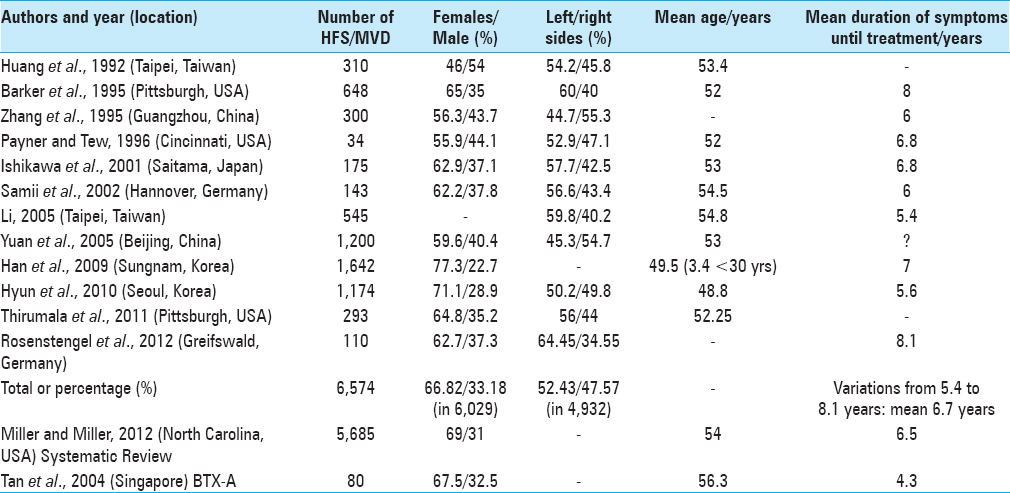
A systematic review of 5,685 cases of HFS submitted to MVD showed 69% were female and 31% were male. Patient mean age at surgery was 54 (45–58) years old. The mean duration of symptoms was 6.5 (4.2–10.7) years.[
Among 6,029 patients with HFS submitted to MVD, 66.82% were female and 33.18% were male [
Cases of bilateral hemifacial spasm in the literature
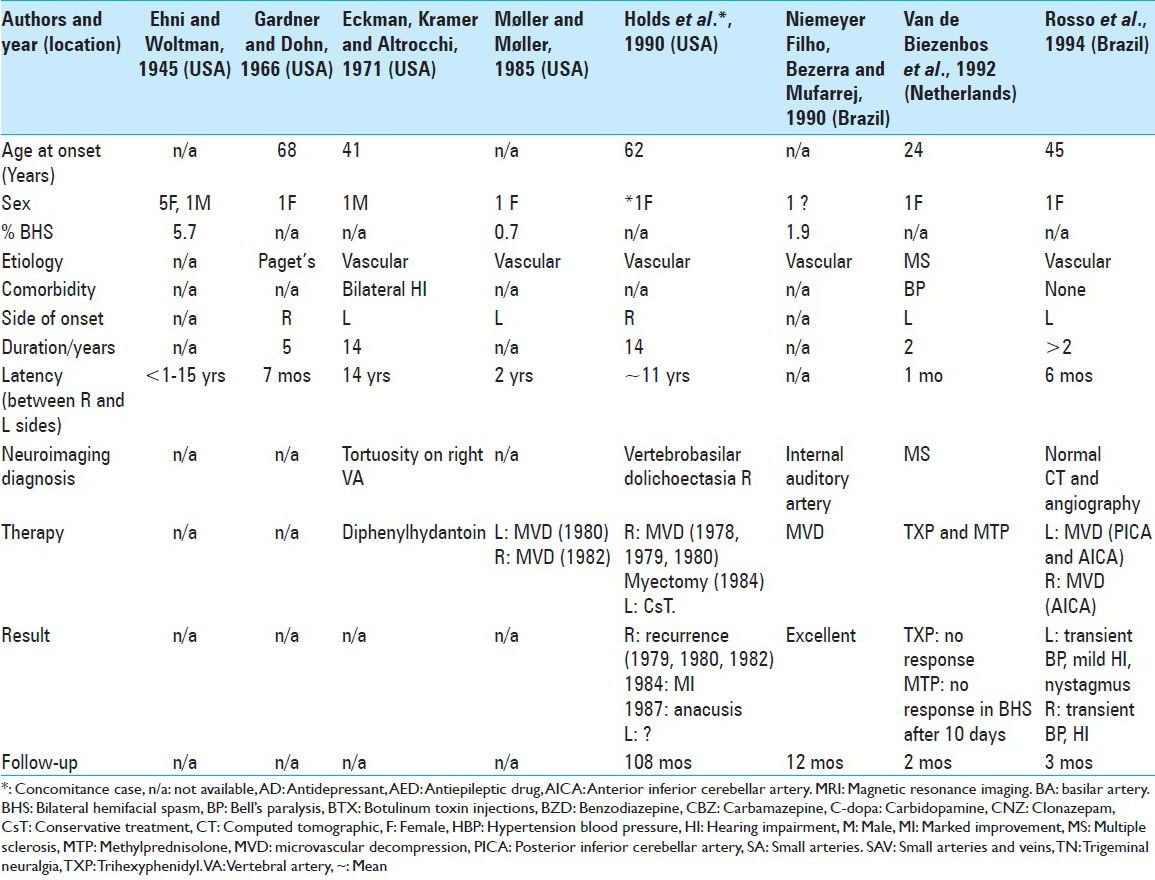
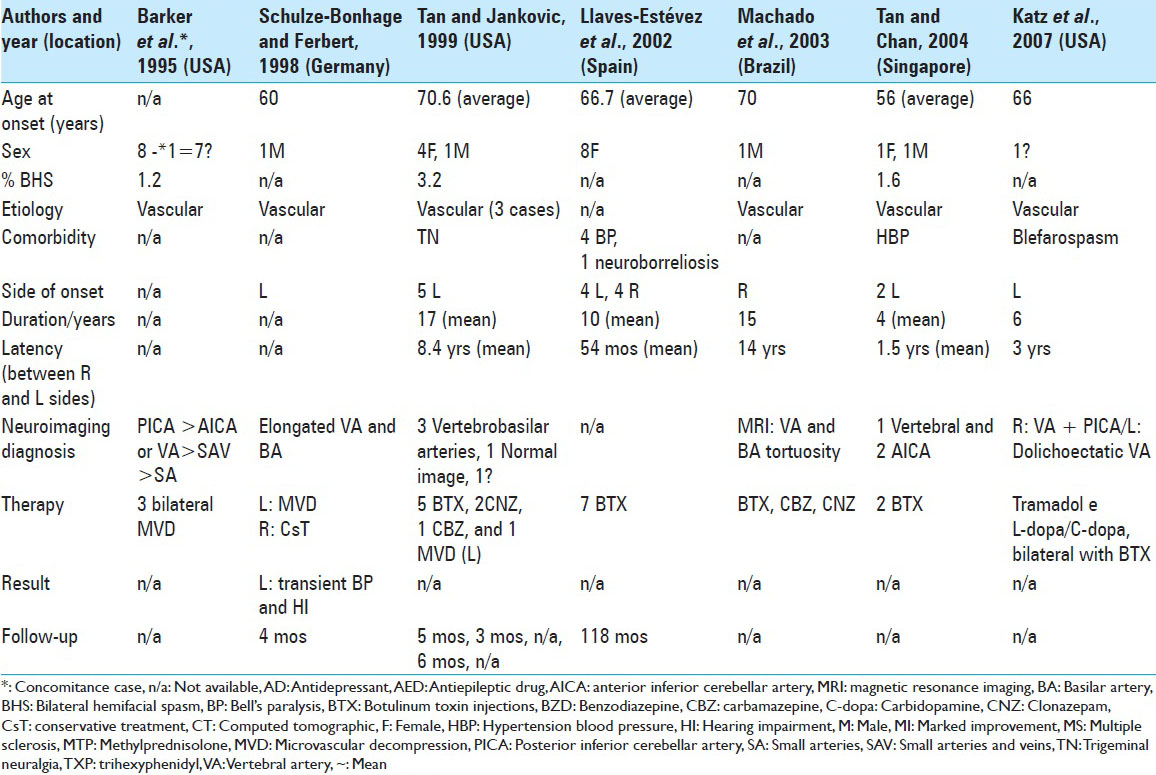
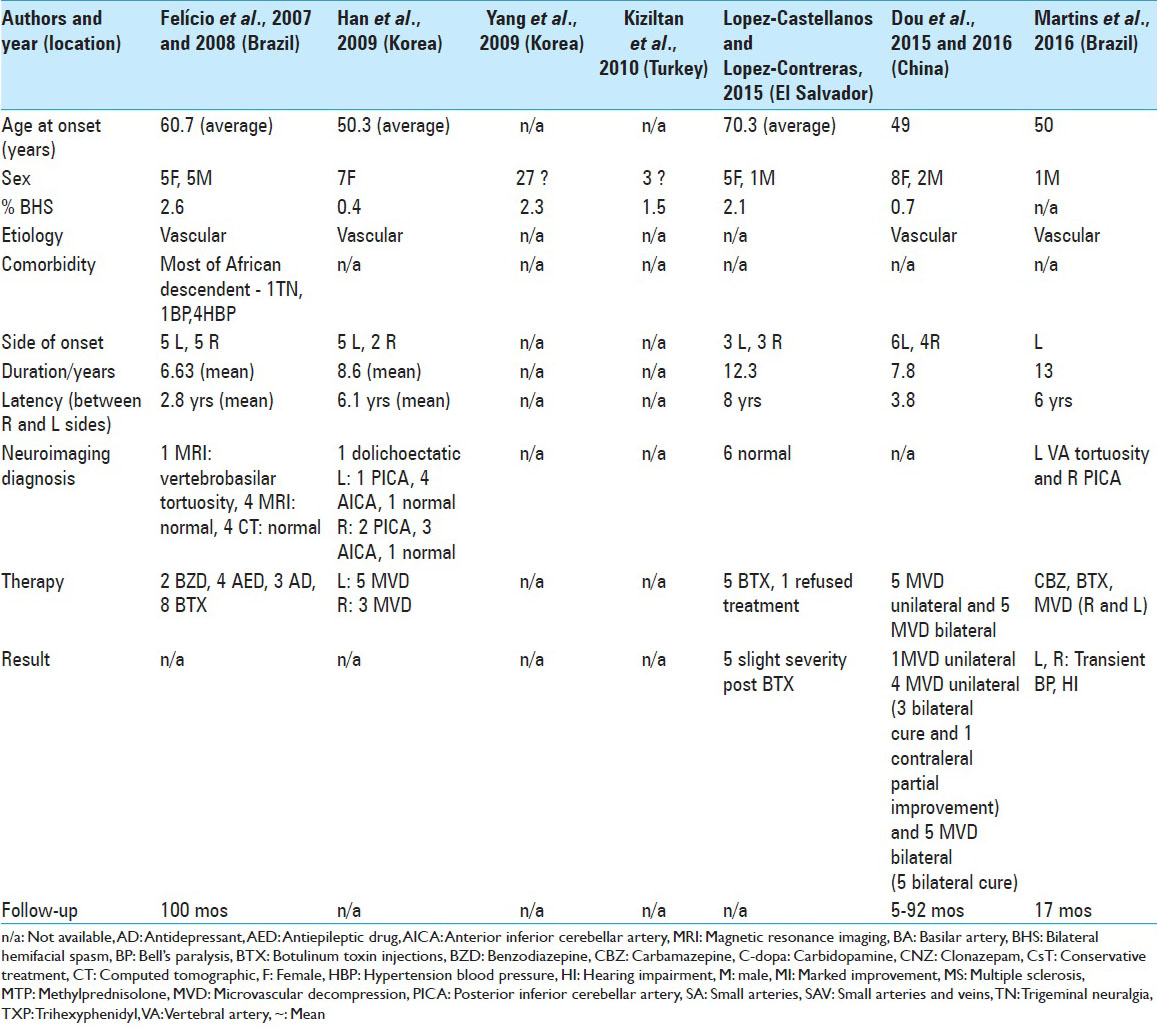
To our knowledge, only 102 cases of BHFS have been reported [
In cases of BHFS, following a highly variable latency period of approximately 33–100 months, the spasms, which initially begin in one hemiface, progress to the contralateral side.[
In series studying HFS, there is a low incidence of BHFS ranging from 0.0% to 5.7% of cases.[
In our analysis, the mean age of onset of symptoms in BHFS was 58.9 years old, with wide variation between 13 years and 88 years old. The predominance between the sexes in 63 cases, for which data were available, was 76% female and 24% male. Symptoms began in the right hemiface in 36% of these cases and on the left in 64%.
Etiology of hemifacial spasm
In a 2012 review of 5,685 cases of HFS submitted to MVD, the NVC frequencies most observed were: 37% by AICA (anterior inferior cerebellar artery), 30% by PICA, and 23% by multiple vessels.[
It is worth noting that the largest series of HFS cases submitted to MVD are Asian, with the exception of the series studied by Jannetta's research group, a US reference in MVD. On the other hand, an Asian cohort did not show higher prevalence of HFS in relation to other ethnic groups.[
Dou et al. (2014)[
Secondary causes of HFS are described as 0.0–0.6% of cases in published series,[
Familial HFS due to probable autosomal transmission with low penetration has been reported.[
The causes of HFS vary, but they are all consequences of changes in motor neuron conduction in the nucleus of the facial nerves by peripheral or central deafferentation and may include cortical and subcortical influences.
Pathophysiology: Historical perspective and current concepts
In 1890, Gilbert, Cadiot, and Roger (apud Meige, 1907)[
In 1898, Habel et al.[
The spread of action potentials in two adjacent unmyelinated axons has been demonstrated in experimental studies, by Katz and Schmitt (1940),[
In 1962, Gardner and Sava,[
Gardner and Dohn (1966)[
The main cause of HFS is NVC in the REZ, which is more vulnerable because this area is devoid of epineurium and Schwann cells because it is only covered by arachnoid cells.[
De Ridder et al.[
Studies involving intraoperative electromyography (EMG) indicated the presence of abnormal muscle responses (AMR) in the facial muscles, such as the lateral spread response (LSR), which were attributed to demyelination/axonal injury of the facial nerve, causing ephaptic transmissions and ectopic excitations, leading to hyperexcitability of facial nucleus motoneurons.[
Experimental studies in rats have demonstrated that facial motoneurons are involved in HFS after facial nerve demyelination. It has been suggested that when a branch of the facial nerve is stimulated, the stimuli are transmitted in axons in both directions, antidromically and orthodromically. They argued that, to induce facial hyperactivity, the phenomena of facial nerve demyelination and vascular compression are both required.[
Research has shown that the severity of facial nerve NVC is associated with spasm intensity.[
The hypothesis of the peripheral origin of facial spasm suggests that ectopic firing and peripheral ephaptic transmissions that originate in their REZ due to NVC and demyelination cause hyperexcitability of the facial nerve nucleus, leading to spasms.[
The hypothesis of the nuclear origin suggests that some form of reorganization of the synapses occurs in the facial nerve nucleus, leading to motor neuron hyperexcitability due to demyelination caused by NVC.[
Thus, neither the nuclear nor the peripheral hypotheses can be rejected. A study involving EMG in a case of BHFS, with asynchronous contractions in each hemiface and treated unilaterally, showed complete resolution of ipsilateral spasms and the persistence of spasms on the contralateral side. This finding suggest independent trigger mechanisms in both hemifaces, more likely due to bilateral nerve zone irritation by aberrant vessels than hyperexcitability of a facial nerve nucleus spreading to the contralateral side.[
A thought-provoking study[
A study involving positron emission tomography (PET scan) in patients with HFS compared with asymptomatic controls showed bilateral glucose hypermetabolism in the somatosensory thalamus [corresponding to the ventral posteromedial (VPM) nucleus, related to the sensory somatotopic part of the face] in patients with HFS, in both active and suppressive states. It is worth noting that the upper part of the face receives motor afferents from ipsilateral and contralateral facial nerves. The spasms were significantly reduced following suppression with BTX-A, according to the Jankovic disability rating scale. Thalamic hypermetabolism was attributed to various causes, such as skin afferents and neuromuscular spindles, antidromic conduction of the facial nerve and secondary changes in the CNS.[
It has been reported that in patients with unilateral writer's cramp, functional MRI (f-MRI) showed increased activity in both putamens, the caudate nuclei, the internal globus pallidus, and the thalamus during tactile stimulation of the right index finger. During dystonias, the basal ganglia-thalamo-cortical motor circuit can occasionally be bilaterally activated by hemilateral stimuli. Blepharospasm and HFS seem to show similar pathophysiology, which explains the bilateral activation of the thalamus by a unilateral stimulus.[
As mentioned above, Gardner and Dohn (1966)[
There are connections between the trigeminal nerve and the facial nerve, which can be demonstrated by the blinking reflex in response to stimulus in the cornea.[
Research has shown the activation of primary and supplementary motor areas by evoked potentials during voluntary eyelid movement,[
The study of transcranial magnetic stimulation (TMS) showed cortical influence on HFS, which, through cortical mechanisms, such as postexcitatory inhibition of motoneurons after excitation of the corticonuclear tract, may activate the corticonuclear inhibitory system controlled by intracortical mechanisms modulated by basal and thalamus ganglia. The facial motoneurons respond easily to trigeminal stimulation, they presumably receive only mild or short-lasting excitation of the corticobulbar inhibitory descending projections.[
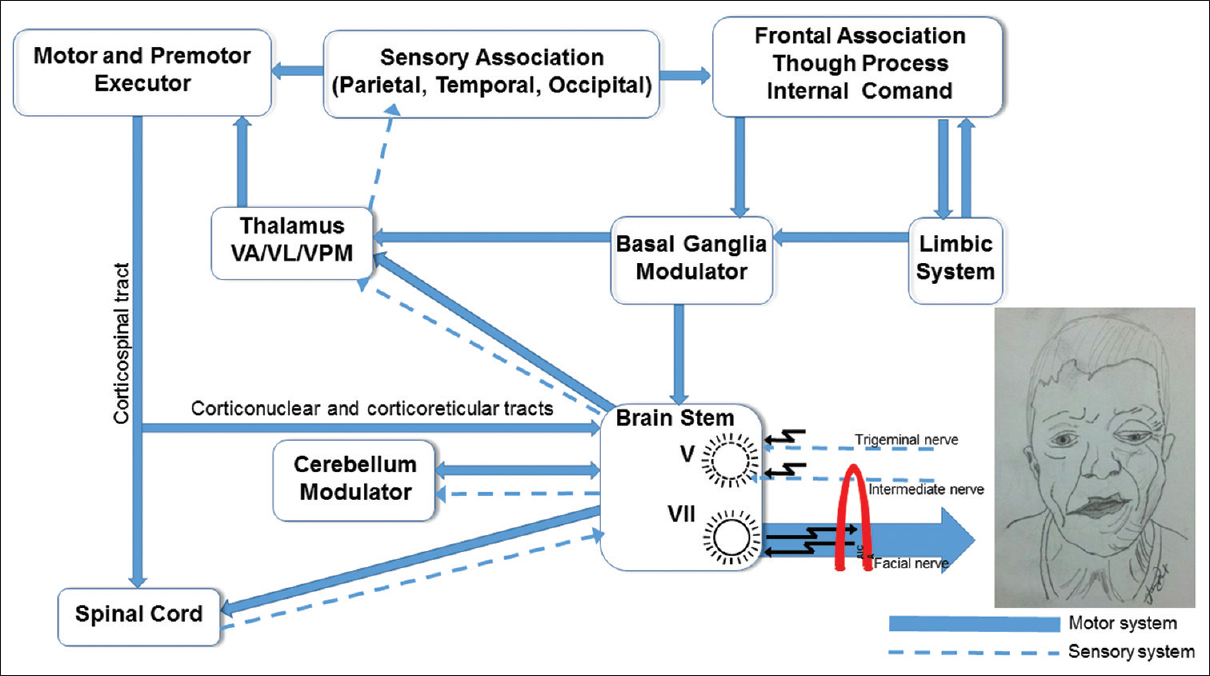
Considering the data outlined here, we argue that the most probable explanation is ectopic firing and ephaptic transmissions, originating in the REZ of the facial nerve due to NVC, orthodromically stimulate the facial muscles and antidromically stimulate the facial nerve nucleus, while the latter, in its hyperexcited state, also intermittently stimulates the facial muscles. These activated muscles trigger the somatosensory afferent impulses of the skin and neuromuscular spindles of the face conducted by the trigeminal nerve, which after passing through the gasserian ganglion and trigeminal nuclei, reach the contralateral VPM somatosensory thalamic nucleus, which then drive to the cortical somesthetic area of the face.
Once activated, this area stimulates the primary and supplementary motor areas through the extrapyramidal system, while the basal ganglia retrogradely activate the motor neurons of the facial nerve nucleus, which peripherally stimulates the facial muscles. In addition, the intermediate nerve, which is anatomically aligned with the facial nerve, likely participates in this pathophysiology. Its impulses orthodromically drive to the spinal trigeminal nucleus and ascend to the basal ganglia-thalamocortical circuits, whereby descending feedback activates the facial nerve nucleus, expressed peripherally through spasms [
It is worth emphasizing that the patient cannot suppress the spasms voluntarily and that they persist during sleep and anesthesia, a fact verified in this case, clearly indicating the influence of the extrapyramidal system and the basal ganglia.
Patients with central facial paralysis, who cannot voluntarily move the muscles of the lower face, show involuntarily movement through reflexes, in response to emotional stimuli from the basal ganglia to the facial nerve nuclei.[
Clinical presentation
Although HFS is not life-threatening, it can cause major disruptions to daily life activities such as driving and working, causing withdrawal from socializing and even depressive disorders.[
Contractions may progress, with predominance of the tonic component over the clonic.[
The spasms begin in adult life, around 45 years of age, and prior history of peripheral facial paralysis or traumatic nerve injury is not uncommon.[
The ipsilateral facial paralysis preceded 1.9% of 106 cases of HFS studied by Ehni and Woltman (1945),[
Previous auditory deficit is also not uncommon in HFS patients submitted to MVD. Huang et al. (1992)[
Differential diagnosis
A diagnosis of BHFS requires considerable care, since it can be mistaken for other facial dyskinesias:
Blepharospasm consists of symmetrical, synchronous, and bilateral contractions of the orbicularis oculi muscle, and may be associated with contractions of the frontalis muscles and the corrugator and procerus muscles, resulting in characteristic depression of the eyebrows (Charcot's sign).[ Facial tics usually begin in childhood, though their characteristics can change, they can be controlled voluntarily and they do not improve with rest. Besides the facial nerve, other nerve groups may be involved, a finding observed in younger patients. The movements are abrupt and multifocal. They are associated with motor, phonic, gestural tics, or other signs and symptoms of Tourette's syndrome[ Facial myokymia is characterized by unilateral and temporary contraction of the orbicularis oculi muscle, usually associated with periods of physical or emotional stress, fatigue, or excessive caffeine consumption[ Synkinesis following facial nerve paralysis leads to the activation of several muscles innervated by the facial nerve and it typically occurs in the context of voluntary movement[ Oromandibular dystonia is usually bilateral and affects other muscular groups not innervated by the facial nerve, such as the pterygoid, masseter, and temporalis muscles[ Hemimasticatory spasm affects the musculature involved with the trigeminal nerve with painful contraction of the medial pterygoid, masseter, and temporalis muscles[ Psychogenic facial spasm does not present a specific pattern of contractions, intensity, and frequency. It can involve muscles not related to the facial nerve and can be modified by distraction Drug-induced late-onset dyskinesias, mainly by neuroleptics, manifest as stereotypical orofacial, lingual and limb movements, and akathisia[ Focal seizures characterized by rapid clonic contractions that can be observed in an EEG or an EEG video.[
The long latency period that occurs between the onset of HFS and contralateral involvement is symptomatic and assists in the differential diagnosis of BHFS and other movement disorders.[
Comorbidities
Arterial hypertension (AH) is the main HFS-related comorbidity, occurring in 24% of cases in a series of 648 patients, and 18.9% in another series of 587 patients.[
Gardner and Dohn (1966)[
Complementary examinations
MRA and MRI are important for evaluating neurovascular conflicts, observed in 15% of HFS cases, or the presence of an expansive lesion at the cerebellopontine angle. A lack of visible neurovascular conflict is often due to limitations in the imaging method.[
EMG is characterized by discharge patterns of spasm activities that can occur as single isolated, clustered, and tonic spasms. Isolated discharges are more prevalent in patients with HFS with shorter disease duration, though over time, tonic spasms tend to prevail. In patients with BHFS, the spasms are asynchronous and asymmetric and the patterns of spasms activities are different.[
Audiometry should be performed to ensure pre- and postoperative comparisons.[
Treatment
To treat HFS, oral medication, BTX-A, and MVD have all been described. Treatment with oral medications is generally considered unsatisfactory. The most commonly prescribed drugs are carbamazepine, phenytoin, clonazepam, baclofen, and gabapentin.[
Acupuncture was prescribed for 85 patients in a series of 310 cases and for 65 patients in another series of 545 cases with HFS, but showed no therapeutic benefits.[
BTX-A applications are a practical therapy that has low morbidity and good clinical response. In general, BTX-A is injected into the affected muscles, resulting in presynaptic blockade of the motor plate that provides a good therapeutic response. The patient remains free from symptoms for 3–4 months, after which it must be reapplied.[
This treatment is the first option in patients with high anesthetic risk, who present milder or refractory symptoms or in those who present HFS relapse following MVD. In cases of BHFS after unilateral MVD, in which hearing loss is a complication, the use of contralateral BTX-A is an excellent option to avoid the risk of bilateral hearing loss, which has high morbidity. Wang and Jankovic (1998)[
The best long-term therapeutic response for HFS due to NVC is endoscope[
Intraoperative EMG monitoring has shown the immediate disappearance of AMR or LSR recorded from a muscle innervated by the superior branch of facial nerve when the inferior branch was stimulated, or conversely, during MVD. This phenomenon is probably due to ephaptic transmission at the lesion site or in combination with motor nucleus hyperactivity.[
One study reported that direct stimulation of the offending artery during surgery in cases of HFS produces a wave analogous to AMR, denominated the “Z-L response,” which disappeared immediately after decompression. Monitoring the Z-L response is useful when an AMR is not recorded prior to decompression or if it persists after all vascular compressions are properly treated. It can help determine the real culprit when multiple offending vessels exist.[
The duration until complete cure of HFS after MVD was studied in 175 Japanese patients, who were assigned to two postoperative groups: Group I (n = 88), in which the residual spasms resumed on day 4, after a period of silence, and diminished gradually until complete resolution in 28 days, on average; and group II (n = 87), in which the spasms ceased immediately after surgery. In group I, 10 (11%) patients did not present a period of silence. Complete resolution of the spasms took more than a year in six (7%) group I patients, with two patients presenting long delays, one of 22 months and another of 27 months. Facial paresis is common in the postoperative period, however, no case of permanent facial paralysis was observed. The authors concluded that approximately 50% of patients show persistent postoperative spasms after a 4-day period of silence, with complete resolution occurring in 25% of cases in 1 week, 50% in 1 month, and 90% within 8 months postsurgery. If the spasms do recur after 4 days and there is no facial paresis, recurrence is more likely and the patient may be considered for surgical re-exploration.[
The mechanism of spasm improvement depends on two factors. The primary mechanism is due to the interruption of ectopic excitation and the efficient transmission that occurs following REZ decompression, leading to the abrupt disappearance of the spasms, in most cases. The secondary mechanism is due to remyelination of the affected segment, with the disappearance of LSR, and perhaps the gradual decrease of neuronal hyperexcitability in the facial nucleus, which could explain delayed improvement.[
The persistence of spasms in the immediate postoperative period varies widely in the literature: 50.3% (Ishikawa et al., 2001),[
Intraoperative brainstem auditory evoked potential (BAEP) monitoring helps prevent auditory deficit.[
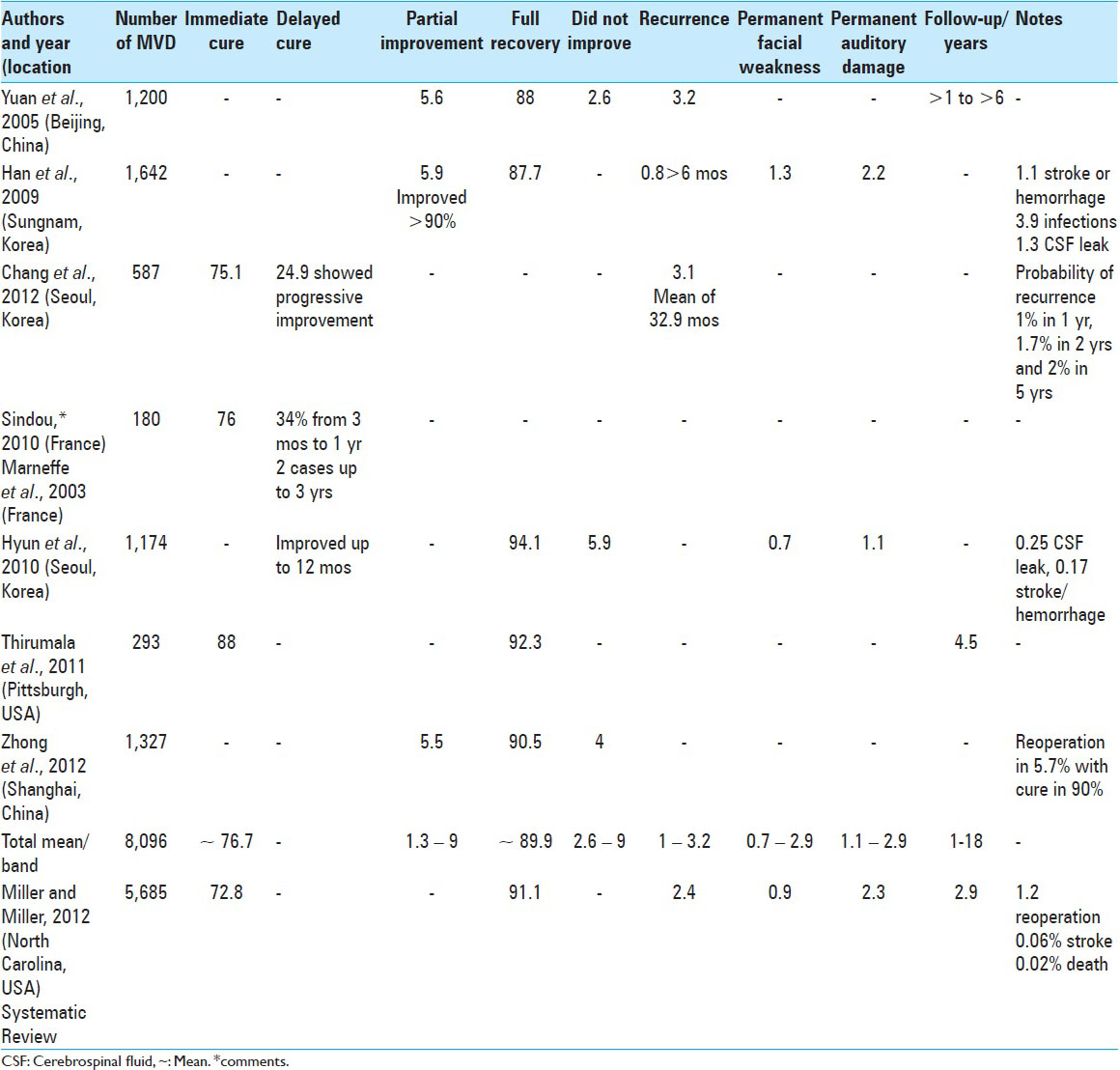
In 2012, a systematic review of 5,685 HFS cases submitted to MVD verified complete cessation of spasms in 91.1% of the cases. The mean follow-up was 2.9 years. Spasms recurred in 2.4% of patients and 1.2% were submitted to a second MVD. Transient complications included 9.5% facial deficit, 3.2% auditory deficit, and 1.4% cerebrospinal fluid fistula. Permanent complications included 2.3% auditory deficit, 0.9% facial paralysis, 0.06% stroke, and 0.02% death. Curiously, the second author of this article had HFS for 5 years, which was cured with MVD.[
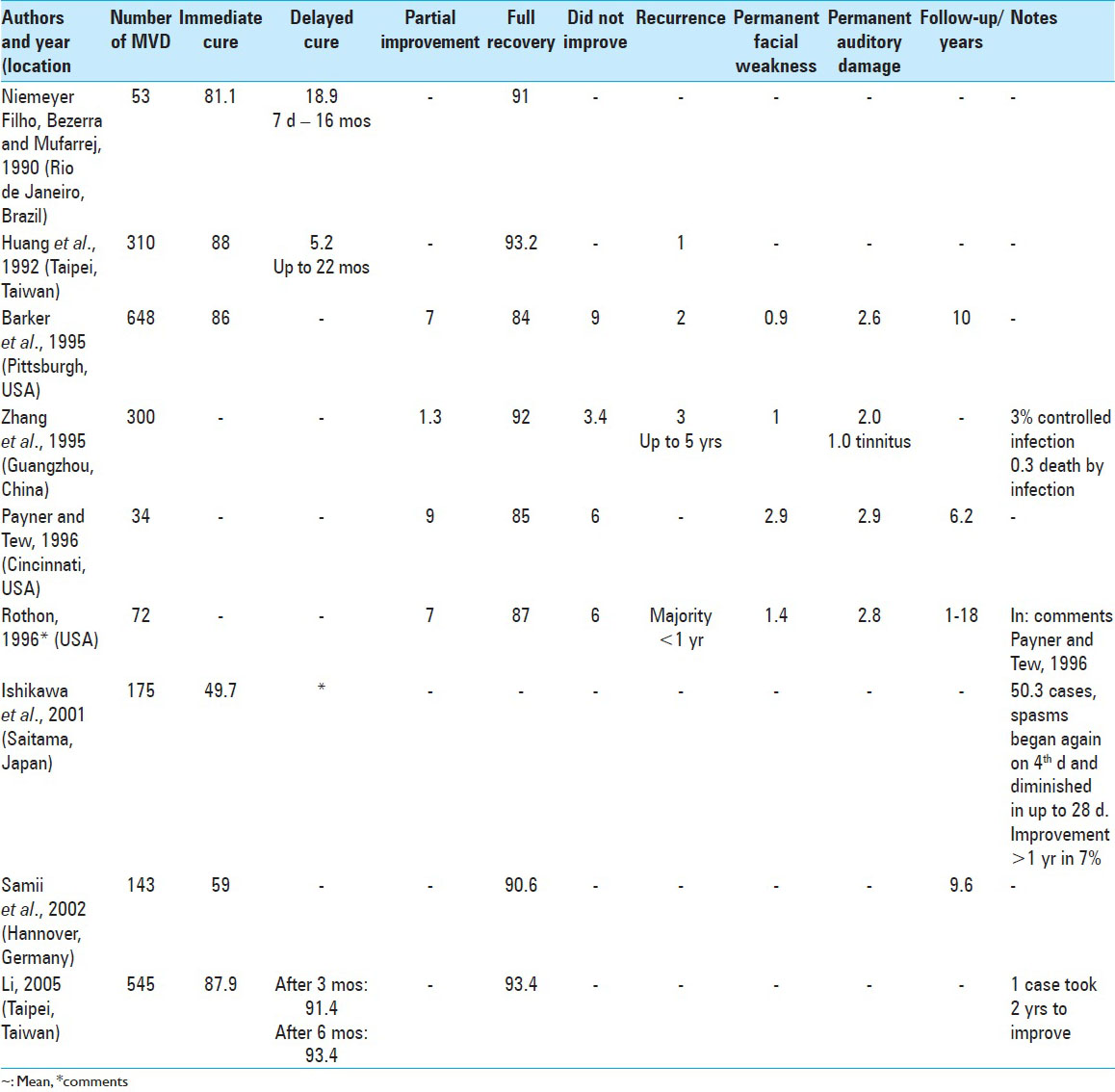
Treatment of HFS by MVD is curative in approximately 90% of cases in the long term and is therefore the best definitive treatment. Improvement immediately following surgery was observed in 76.7% of cases, on average, with the majority of delayed improvements resolving within a year. Partial improvement varied from 1.3% to 9% and the total failure of MVD ranged from 2.6% to 9%. Cases of recurrence ranged from 1% to 3.2% in follow-up ranging from 1 year to 18 years. Permanent hearing loss ranged from 1.1% to 2.9% and permanent facial deficit ranged from 0.7% to 2.9% of cases. There was only one case (0.01%) of mortality due to infection in 8,096 cases of HFS submitted to MVD [
Special secondary cases of HFS can be treated with other techniques such as embolization, when treating arteriovenous malformations, gamma knife surgery, or microsurgery. All of these methods are effective.[
CONCLUSIONS
BHFS is a rare neurological syndrome. Diagnosis is a challenge that depends on excluding other facial dyskinesias. We present the tenth case of BHFS, which was resolved with bilateral surgery. Microvascular decompression is a definitive, long-term surgical option with low complications.
In cases of BHFS, we believe that the best approach is bilateral MVD. If hearing loss occurs following a unilateral surgery, the use of contralateral BTX-A is the best option to avoid the risk of bilateral hearing loss, a complication of considerable morbidity. Use of BTX-A prior to other treatments should be considered in patients with a high anesthetic risk, when the symptoms in one hemiface are mild, to avoid surgery on that side, or at the patient's discretion.
The described case was successfully submitted to bilateral MVD after attempting to treat the patient with oral medications and BTX-A, which ended in relapses.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Afifi AK, Bergman RA.editorsFunctional Neuroanatomy: Text and Atlas. New York: McGraw-Hill Education; 2004. p.
2. Arvanitaki A. Effects evoked in an axon by the activity of a contiguous one. J Neurophysiol. 1942. 5: 89-108
3. Auger RG, Piepgras DG, Laws ER. Hemifacial spasm: Results of microvascular decompression of the facial nerve in 54 patients. Mayo Clin Proc. 1986. 61: 640-4
4. Auger RG, Whisnant JP. Hemifacial spasm in Rochester and Olmsted County, Minnesota, 1960 to 1984. Arch Neurol. 1990. 47: 1233-4
5. Barbosa ER, Costa MDL, Staut CC, Bacheschi LA, Bittar MS. Espasmo hemifacial familiar: Relato de dois casos. Arq Neuropsiquiatr. 1998. 56: 111-5
6. Barker FG, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD. Microvascular decompression for hemifacial spasm. J Neurosurg. 1995. 82: 201-10
7. Carter JB, Patrinely JR, Jankovic J, McCrary JA, Boniuk M. Familial hemifacial spasm. Arch Ophthalmol. 1990. 108: 249-50
8. Chang WS, Chung JC, Kim JP, Chung SS, Chang JW. Delayed recurrence of hemifacial spasm after successful microvascular decompression: Follow-up results at least 5 years after surgery. Acta Neurochir. 2012. 154: 1613-9
9. Cheng WY, Chao SC, Shen CC. Endoscopic microvascular decompression of the hemifacial spasm. Surg Neurol. 2008. 70: 40-6
10. De Ridder D, Møller A, Verlooy J, Cornelissen M, De Ridder L. Is the root entry/exit zone important in microvascular compression syndromes?. Neurosurgery. 2002. 51: 427-34
11. Defazio G, Berardelli A, Abbruzzese G, Coviello V, De Salvia R, Federico F. Primary hemifacial spasm and arterial hypertension: A multicenter case–control study. Neurology. 2000. 54: 1198-200
12. Devoize JL. “The other” Babinski's sign: Paradoxical raising of the eyebrow in hemifacial spasm. J Neurol Neurosurg Psychiatry. 2001. 70: 516-
13. Dou NN, Xia L, Liu MX, Zhong J. Bilateral hemifacial spasm might be cured by unilateral microvascular decompression. Acta Neurochir. 2015. 157: 467-8
14. Dou NN, Zhong J, Liu MX, Xia L, Sun H, Li B. Management of bilateral hemifacial spasm with microvascular decompression. World Neurosurg. 2016. 87: 640-5
15. Dou NN, Zhong J, Zhou QM, Zhu J, Wang YN, Xia L. The mechanism of hemifacial spasm: A new understanding of the offending artery. Neurol Res. 2015. 37: 184-8
16. Eckman PB, Kramer RA, Altrocchi PH. Hemifacial spasm. Arch Neurol. 1971. 25: 81-7
17. Ehni G, Woltman HW. Hemifacial spasm. Review of one hundred and six cases. Arch NeurPsych. 1945. 53: 205-11
18. Felicio AC, Godeiro CDO, Borges V, Silva SMD, Ferraz HB. Bilateral hemifacial spasm and trigeminal neuralgia: A unique form of painful tic convulsif. Mov Disord. 2007. 22: 285-6
19. Felicio AC, Godeiro-Junior CD, Borges V, Silva S, Ferraz HB. Bilateral hemifacial spasm: A series of 10 patients with literature review. Parkinsonism Relat D. 2008. 14: 154-6
20. Ferguson JH. Hemifacial spasm and facial nucleus. Ann Neurol. 1978. 4: 97-103
21. Fu KK, Ko A. The treatment with alendronate in hemifacial spasm associated with Paget's disease of bone. Clin Neurol Neurosurg. 2000. 102: 48-51
22. Fukushima T, Sindou M, Matsushima T. Microvascular decompression for treating hemifacial spasm: Lessons learned from a prospective study of 1,174 operations. Comments. Neurosurg Rev. 2010. 33: 334-
23. Garcia M, Naraghi R, Zumbrunn T, Rösch J, Hastreiter P, Dörfler A. High-Resolution 3D-Constructive Interference in Steady-State MR Imaging and 3D Time-of-Flight MR Angiography in Neurovascular Compression: A Comparison between 3T and 1.5T. Am J Neuroradiol. 2012. 33: 1251-6
24. Gardner WJ, Dohn DF. Trigeminal neuralgia-hemifacial spasm-Paget's disease: Significance of this association. Brain. 1966. 89: 555-62
25. Gardner WJ, Sava GA. Hemifacial spasm - a reversible pathophysiologic state. J Neurosurg. 1962. 19: 240-7
26. Gobbele R, Halboni P, Buchner H, Waberski TD. Interference of tactile and pain stimuli on thalamocortical signal processing in humans revealed by median nerve SEPs. Clin Neurophysiol. 2007. 118: 2497-505
27. Gordon A. Convulsive movements on the face: Differential diagnosis; effect alcoholic injections. JAMA. 1912. LVIII: 97-102
28. Gowers WR, Gowers WR.editors. Diseases of the cranial Nerves. A Manual of Diseases of the Nervous System. London: J & A Churchill; 1886. p.
29. Granit R, Leksell L, Skoglund CR. Fibre interaction in injured or compressed region of nerve. Brain. 1944. 67: 125-40
30. Habel A. Ueber Fortbestehen von Tic convulsif bei gleichseitiger Hemiplegie. Deutsche Med Wchnschr. 1898. 24: 189-
31. Han IB, Chang JH, Chang JW, Huh R, Chung SS. Unusual causes and presentations of hemifacial spasm. Neurosurgery. 2009. 65: 130-7
32. Hiwatashi A, Yoshiura T, Yamashita K, Kamano H, Honda H. High-Resolution STIR for 3-T MRI of the Posterior Fossa: Visualization of the Lower Cranial Nerves and Arteriovenous Structures Related to Neurovascular Compression. AJR Am J Roentgenol. 2012. 199: 644-8
33. Holds JB, Anderson RL, Jordan DR, Patrinely JR. Bilateral hemifacial spasm. J Clin Neuroophthalmol. 1990. 10: 153-4
34. Huang CI, Chen IH, Lee LS. Microvascular decompression for hemifacial spasm-Analyses of operative findings and results in 310 patients. Neurosurgery. 1992. 30: 53-7
35. Huizar P, Kuno M, Miyata Y. Electrophysiological properties of spinal motoneurones of normal and dystrophic mice. J Physiol. 1975. 248: 231-46
36. Hunt J. The sensory system of the facial nerve and its symptomatology. J Nerv Ment Dis. 1909. 36: 321-50
37. Hyun SJ, Kong DS, Park K. Microvascular decompression for treating hemifacial spasm: Lessons learned from a prospective study of 1,174 operations. Neurosurg Rev. 2010. 33: 325-34
38. Ishikawa M, Nakanishi T, Takamiya Y, Namiki J. Delayed resolution of residual hemifacial spasm after microvascular decompression operations. Neurosurgery. 2001. 49: 847-54
39. Ishikawa M, Ohira T, Namiki J, Gotoh K, Takase M, Toya S. Electrophysiological investigation of hemifacial spasm: F-waves of the facial muscles. Acta Neurochir. 1996. 138: 24-32
40. Ishikawa M, Ohira T, Namiki J, Kobayashi M, Takase M, Kawase T. Electrophysiological investigation of hemifacial spasm after microvascular decompression: F waves of the facial muscles, blink reflexes, and abnormal muscle responses. J Neurosurg. 1997. 86: 654-61
41. Jannetta PJ, Hackett E, Ruby JR. Electromyographic and electron microscopic correlates in hemifacial spasm treated by microsurgical relief of neurovascular compression. Surg Forum. 1970. 21: 449-51
42. Katter JT, Dado RJ, Kostarczyk E, Giesler GJ. Spinothalamic and spinohypothalamic tract neurons in the sacral spinal cord of rats. I. Locations of antidromically identified axons in the cervical cord and diencephalon. J Neurophysiol. 1996. 75: 2581-605
43. Katz B, Schmitt OH. Electrical interaction between two adjacent nerve fibres. J Physiol. 1940. 97: 471-88
44. Katz BJ, Burroughs JR, Anderson RL, Bownds S, McCann JD. Asynchronous blepharospasm, facial and cervical dystonia, and bilateral asynchronous hemifacial spasm. Mov Disord. 2007. 22: 231-4
45. Kiernan JA.editorsNeuroanatomia Humana de Barr. Barueri: Manole; 2003. p.
46. Kim P, Fukushima T. Observations on synkinesis in patients with hemifacial spasm - effect of microvascular decompression and etiological considerations. J Neurosurg. 1984. 60: 821-7
47. Kiziltan ME, Sahin R, Akalin MA, Uzun N, Savrun FK. Discharge patterns of the spasm activity in hemifacial spasm: A summary of 206 patients. Nobel Med. 2010. 6: 9-14
48. Konan AV, Roy D, Raymond J. Endovascular treatment of hemifacial spasm associated with a cerebral arteriovenous malformation using transvenous embolization: Case report. Neurosurgery. 1999. 44: 663-6
49. Kong DS, Park K. Hemifacial spasm: A neurosurgical perspective. J Korean Neurosurg Soc. 2007. 42: 355-62
50. Kotterba S, Tegenthoff M, Malin JP. Hemifacial spasm or somatoform disorder – Postexcitatory inhibition after transcranial magnetic cortical stimulation as a diagnostic tool. Acta Neurol Scand. 2000. 101: 305-10
51. Kuroki A, Møller AR. Facial-nerve demyelination and vascular compression are both needed to induce facial hyperactivity - a study in rats. Acta Neurochir. 1994. 126: 149-57
52. Lagalla G, Logullo F, Di Bella P, Haghighipour R, Provinciali L. Familial hemifacial spasm and determinants of late onset. Neurol Sci. 2010. 31: 17-22
53. Lee MH, Jee TK, Lee JA, Park K. Postoperative complications of microvascular decompression for hemifacial spasm: Lessons from experience of 2040 cases. Neurosurg Rev. 2016. 39: 151-8
54. Li CS. Varied patterns of postoperative course of disappearance of hemifacial spasm after microvascular decompression. Acta Neurochir. 2005. 147: 617-20
55. Llaves-Estevez L, Chacon-Pena J, Martinez-Fernandez E, Burguera-Hernandez JA, Valero C. Bilateral hemifacial spasm: Eight personal case reports. Rev Neurol. 2002. 35: 401-3
56. Lopez-Castellanos JR, Lopez-Contreras JR. Bilateral hemifacial spasm: Report of 6 cases in El Salvador. Mov Disord. 2015. 30: S511-2
57. Machado FCN, Fregni F, Campos CR, Limongi JCP. Bilateral hemifacial spasm: Case report. Arq Neuropsiquiatr. 2003. 61: 115-8
58. Magnan J, Caces F, Locatelli P, Chays A. Hemifacial spasm: Endoscopic vascular decompression. Otolaryngol Head Neck Surg. 1997. 117: 308-14
59. Marneffe V, Polo G, Fischer C, Sindou M. Microsurgical vascular decompression for hemifacial spasm. Follow-up over one year, clinical results and prognostic factors. Study of a series of 100. cases. Neurochirurgie. 2003. 49: 527-35
60. McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK. Microvascular decompression of cranial nerves: Lessons learned after 4400 operations. J Neurosurg. 1999. 90: 1-8
61. Meige H, Feindel E.editorsTics and Their Treatment. New York: William Wood and Company; 1907. p.
62. Micheli F, Scorticati MC, Raina G. Beneficial effects of botulinum toxin type a for patients with painful tic convulsif. Clin Neuropharmacol. 2002. 25: 260-2
63. Miller LE, Miller VM. Safety and effectiveness of microvascular decompression for treatment of hemifacial spasm: A systematic review. Br J Neurosurg. 2012. 26: 438-44
64. Møller AR. Vascular compression of cranial nerves: II: Pathophysiology. Neurol Res. 1999. 21: 439-43
65. Møller AR, Jannetta PJ. Monitoring facial EMG responses during microvascular decompression operations for hemifacial spasm. J Neurosurg. 1987. 66: 681-5
66. Møller AR, Jannetta PJ. On the origin of synkinesis in hemifacial spasm - results of intracranial recordings. J Neurosurg. 1984. 61: 569-76
67. Møller AR. The cranial nerve vascular compression syndrome: II. A review of pathophysiology. Acta Neurochir. 1991. 113: 24-30
68. Møller MB, Møller AR. Loss of auditory function in microvascular decompression for hemifacial spasm. J Neurosurg. 1985. 63: 17-20
69. Nielsen VK. Electrophysiology of the nerve in hemifacial spams: Ectopic/ephaptic excitation. Muscle Nerve. 1985. 8: 545-55
70. Nielsen VK. Pathophysiology of hemifacial spasm: II. Lateral spread of the supraorbital nerve reflex. Neurology. 1984. 34: 427-31
71. Nielsen VK, Jannetta PJ. Pathophysiology of hemifacial spasm: III. Effects of facial nerve decompression. Neurology. 1984. 34: 891-7
72. Niemeyer Filho P, Bezerra M, Mufarrej G. Espasmo hemifacial resultados da descompressão microvascular em 53 pacientes. Arq Neuropsiquiatr. 1990. 48: 210-6
73. Nilsen B, Le KD, Dietrichs E. Prevalence of hemifacial spasm in Oslo, Norway. Neurology. 2004. 63: 1532-3
74. Obeso JA, Rodriguez MC, DeLong MR, Obeso JA, DeLong MR, Ohye C, Marsden CD, Obeso JA, DeLong MR, Ohye C, Marsden CD.editors. The Basal ganglia and new surgical approaches of Parkinson's disease. Advances in Neurology. Philadelphia: Lippincott-Ravens; 1997. p.
75. Ogawara K, Kuwabara S, Kamitsukasa L, Mizobuchi K, Misawa S, Hattori T. Trigeminal afferent input alters the excitability of the facial motoneurons in hemifacial spasm. Neurology. 2004. 62: 1740-52
76. Oyama H, Ikeda A, Inoue S, Nakashima Y, Shibuya M. Local injection of botulinum toxin type a for hemifacial spasm. Neurol Med Chir. 2002. 42: 245-9
77. Payner TD, Tew JMJ. Recurrence of hemifacial spasm after microvascular decompression. Neurosurgery. 1996. 38: 686-91
78. Peller MZeuner KEMunchau AQuartarone AWeiss MKnutzen A. http://surgicalneurologyint.com/surgicalint-articles/a-tribute-to-atos-de-sousa-md/.
79. Rasninsky M. Physiological properties of dystrophic mouse spinal root axons. Electroencephalogr Clin Neurophysiol Suppl. 1982. 36: 99-105
80. Rosenstengel C, Matthes M, Baldauf J, Fleck S, Schroeder H. Hemifacial spasm conservative and surgical treatment options. Dtsch Arztebl Int. 2012. 109: 667-73
81. Rosso ALZ, Mattos JP, Fogel LM, Novis SAP. Bilateral hemifacial spasm. Mov Disord. 1994. 9: 236-7
82. Ruby JR, Jannetta PJ. Hemifacial spasm: Ultrastructural changes in the facial nerve induced by neurovascular compression. Surg Neurol. 1975. 4: 360-70
83. Russel JSR, Allbutt C, Rolleston HD.editors. Facial Spasm. A System of Medicine. London: Macmillan; 1910. p.
84. Samii M, Gunther T, Iaconetta G, Muehling M, Vorkapic P, Samii A. Microvascular decompression to treat hemifacial spasm: Long-term results for a consecutive series of 143 patients. Neurosurgery. 2002. 50: 712-8
85. Sanders DB. Ephaptic transmission in hemifacial spasm - a single-fiber emg study. Muscle Nerve. 1989. 12: 690-4
86. Schulze-Bonhage A, Ferbert A. Electrophysiological recordings in bilateral hemifacial spasm. J Neurol Neurosurg Psychiatry. 1998. 65: 408-10
87. Sekula RF, Bhatia S, Frederickson AM, Jannetta PJ, Quigley MR, Small GA. Utility of intraoperative electromyography in microvascular decompression for hemifacial spasm: A meta-analysis. Neurosurg Focus. 2009. 27: 1-6
88. Shimizu M, Suzuki Y, Kiyosawa M, Wakakura M, Ishii K, Ishiwata K. Glucose hypermetabolism in the thalamus of patients with hemifacial spasm. Mov Disord. 2012. 27: 519-25
89. Sindou M, Howeidy T, Acevedo G. Anatomical observations during microvascular decompression for idiopathic trigeminal neuralgia (with correlations between topography of pain and site of the neurovascular conflict). Prospective study in a series of 579 patients. Acta Neurochir. 2002. 144: 1-12
90. Skinner HA. Some histologic features of the cranial nerves. Arch NeurPsych. 1931. 25: 356-72
91. Suzuki Y, Kiyosawa M, Mochizuki M, Ishiwata K, Ishii K. The pre-supplementary and primary motor areas generate rhythm for voluntary eye opening and closing movements. Tohoku J Exp Med. 2010. 222: 97-104
92. Tan EK, Chan LL. Clinico-radiologic correlation in unilateral and bilateral hemifacial spasm. J Neurol Sci. 2004. 222: 59-64
93. Tan EK, Fook-Chong S, Lum SY, Lim E. Botulinum toxin improves quality of life in hemifacial spasm: Validation of a questionnaire (HFS-30). J Neurol Sci. 2004. 219: 151-5
94. Tan EK, Jankovic J. Bilateral hemifacial spasm: A report of five cases and a literature review. Mov Disord. 1999. 14: 345-9
95. Tan EK, Jankovic J. Psychogenic hemifacial spasm. J Neuropsychiatry Clin Neurosci. 2001. 13: 380-4
96. Tanrikulu L, Scholz T, Nikoubashman O, Wiesmann M, Clusmann H. Preoperative MRI in neurovascular compression syndromes and its role for microsurgical considerations. Clin Neurol Neurosurg. 2015. 129: 17-20
97. Thirumala PD, Shah AC, Nikonow TN, Habeych ME, Balzer JR, Crammond DJ. Microvascular decompression for hemifacial spasm: Evaluating outcome prognosticators including the value of intraoperative lateral spread response monitoring and clinical characteristics in 293 patients. J Clin Neurophysiol. 2011. 28: 56-66
98. Tokucoglu F, Sucu HK, Celebisoy M, Gelal F. Hemifacial spasm in correlation with electrophysiological and radiological findings. Acta Neurol Bel. 2008. 108: 94-8
99. Vallssole J, Tolosa ES. Blink reflex excitability cycle in hemifacial spasm. Neurology. 1989. 39: 1061-6
100. Vandebiezenbos JBM, Horstink M, Vandevlasakker CJW, Vanengelen BGM, Hommes ORV, Barkhof F. A case of bilateral alternating hemifacial spasms. Mov Disord. 1992. 7: 68-70
101. Velickovic M, Tse W. Bilateral synchronous hemifacial spasm (HFS) after bilateral Bell's palsy (BP). Mov Disord. 2009. 24: S502-
102. Wang AC, Jankovic J. Hemifacial spasm: Clinical findings and treatment. Muscle Nerve. 1998. 21: 1740-7
103. Wilson SAK.editorsNeurology. London: Edward Arnold; 1940. p.
104. Yaltho TC, Jankovic J. The many faces of hemifacial spasm: Differential diagnosis of unilateral facial spasms. Mov Disord. 2011. 26: 1582-92
105. Yang KH, Na JH, Kong DS, Park K. Combined hyperactive dysfunction syndrome of the cranial nerves. J Korean Neurosurg Soc. 2009. 46: 351-4
106. Yuan Y, Wang Y, Zhang SX, Zhang L, Li R, Guo J. Microvascular decompression in patients with hemifacial spasm: Report of 1200 cases. Chin Med J. 2005. 118: 833-6
107. Zhang KW, Shun ZT. Microvascular decompression by the retrosigmoid approach for idiopathic hemifacial spasm-experience with 300 cases. Ann Otol Rhinol Laryngol. 1995. 104: 610-2
108. Zhong J, Li ST, Zhu J, Guan HX, Zhou QM, Jiao W. A clinical analysis on microvascular decompression surgery in a series of 3000 cases. Clin Neurol Neurosurg. 2012. 114: 846-51
109. Zhong J, Zhu J, Sun H, Dou NN, Wang YN, Ying TT. Microvascular decompression surgery: Surgical principles and technical nuances based on 4000 cases. Neurol Res. 2014. 36: 882-93