- Department of Neurosurgery, Karolinska Institute, Centre for Allogeneic Stem Cell Transplantation, Karolinska University Hospital, Stockholm, Sweden
- Department of Medical Radiation Physics and Nuclear Medicine, Karolinska Institute, Centre for Allogeneic Stem Cell Transplantation, Karolinska University Hospital, Stockholm, Sweden
- Division of Therapeutic Immunology, Department of Laboratory Medicine, Karolinska Institute, Centre for Allogeneic Stem Cell Transplantation, Karolinska University Hospital, Stockholm, Sweden
Correspondence Address:
Georges Sinclair
Department of Neurosurgery, Karolinska Institute, Centre for Allogeneic Stem Cell Transplantation, Karolinska University Hospital, Stockholm, Sweden
DOI:10.4103/sni.sni_480_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Georges Sinclair, Hamza Benmakhlouf, Marina Brigui, Markus Maeurer, Ernest Dodoo. The concept of rapid rescue radiosurgery in the acute management of critically located brain metastases: A retrospective short-term outcome analysis. 30-Oct-2018;9:218
How to cite this URL: Georges Sinclair, Hamza Benmakhlouf, Marina Brigui, Markus Maeurer, Ernest Dodoo. The concept of rapid rescue radiosurgery in the acute management of critically located brain metastases: A retrospective short-term outcome analysis. 30-Oct-2018;9:218. Available from: http://surgicalneurologyint.com/surgicalint-articles/9055/
Abstract
Background:Adaptive hypofractionated gamma knife radiosurgery has been used to treat brain metastases in the eloquent regions while limiting the risk of adverse radiation effect (ARE). Ablative responses might be achieved within days to weeks with the goal to preserve the neurological function. The application of this treatment modality in selected acute/subacute settings has been termed Rapid Rescue Radiosurgery (RRR) in our department. We report the expeditious effects of RRR during treatment and 4 weeks after treatment completion.
Methods:In all, 34 patients with 40 brain metastases, each treated over a period of 7 days in three separate gamma knife radiosurgery sessions (GKRS 1-3) between November 2013 and August 2017, were retrospectively analyzed in terms of tumor volume reduction, salvage of organs at risk (OAR), and radiation induced toxicity under the period of treatment (GKRS 1-3 = one week) and at first follow-up magnetic resonance imaging (MRI) (4 weeks after GKRS 3).
Results:Mean tumor volume at GKRS 1 was 12.8 cm3. Mean peripheral doses at GKRS 1, GKRS 2, and GKRS 3 were 7.7 Gy, 8.1 Gy, and 8.4 Gy (range: 6.0-9.5 Gy) at the 35% to 50% isodose lines. In the surviving group at first follow-up (n = 28), mean tumor volume reduction was − 10% at GKRS 3 (1 week) and − 48% four weeks after GKRS 3. There was no further clinical deterioration between GKRS 3 and first follow-up in 21 patients. Six patients died prior to first follow-up due to extracranial disease. No ARE was noticed/reported.
Conclusions:In this study, RRR proved effective in terms of rapid tumor volume reduction, debulking, and preservation/rescue of neurological function.
Keywords: Adaptive hypofractionation, adverse radiation event, brain metastases, gamma knife radiosurgery, recursive partitioning analysis
INTRODUCTION
The management of large brain metastases in or near eloquent brain remains a major challenge in the field of neuro-oncology. These life-threatening lesions often lead to prompt and severe neurological impairment. Their acute and subacute management is difficult, particularly with underlying metachronous/synchronous metastatic activity. In addition, recursive partitioning analysis (RPA)–surrogate factors may limit safe surgical removal and optimal postsurgical focal cavity radiation. Chemotherapeutic approaches are deemed of low efficacy due to blood-brain barrier constraints. Despite promising advances in the field of immunotherapy, exemplified in the ascent of check point inhibitors, there are limited data on the efficacy of immunotherapeutic approaches in the treatment of brain metastases.[
However, single fraction radiosurgery may not be feasible in the face of large metastases due to the risk of adverse radiation effect/event (ARE) development, particularly in eloquent brain. Several reports have presented stereotactic hypofractionation as an alternative to single-fraction radiosurgery, retaining comparative local control with reduced risk of ARE.[
The aim of this first series study is to analyze the effects of RRR in terms of tumor volume reduction dynamics and ensuing decompression during the time of treatment (7-day period) and at 4 weeks after treatment completion.
MATERIALS AND METHODS
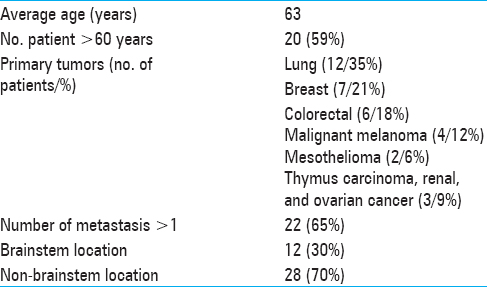
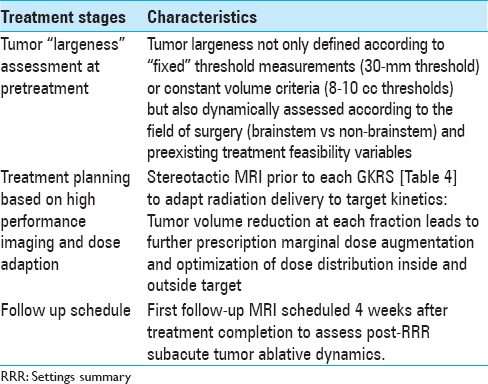
Between November 2013 and August 2017, 34 consecutive patients with 40 cerebral metastases were treated with RRR at our center, the Gamma Knife Unit, Department of Neurosurgery, Karolinska University Hospital, Stockholm. In all cases, the lesions were deemed not suitable for microsurgery, systemic therapy, or other form of radiotherapy. We conducted a retrospective analysis of all medical records and corresponding imaging (treatment and follow-up), including LGP (Leksell GammaPlan) volume data. RRR was stratified in two fields: (i) brainstem radiosurgery which included intrinsic and extrinsic brainstem lesions (B-RRR) and (ii) nonbrainstem radiosurgery (NB-RRR), consisting of intra- and extra-axial metastatic lesions, including dural metastases and focal leptomeningeal metastases. In this study, RRR was applied in the metastatic lesions assessed as “large” and hence not suitable for single fraction gamma knife radiosurgery (SF-GKRS). Traditionally, metastatic lesions have been volumetrically defined as “large” based on straightforward mathematical calculations (generally, >30 mm in diameter and/or >8-10 cm in volume3) regardless of the focal topographic conditions. In the context of RRR settings, the definition of tumor “largeness” was dynamically assessed by considering a number of factors: (i) dose volume estimates at pretreatment and at GKRS 1 (intra- and extra-tumoral dose distributions in relation to the single and multiple fraction treatment), (ii) LQ model–based isoeffective dose conversions, and (iii) treatment feasibility variables (TFV). The latter variables were identified as follows:
Affected brain regions: degree of regional eloquence and corresponding neurologic function Location and the number of organs at risk Presence of perilesional edema Prior radiation therapy with potential/synergic impact on future ARE-evolvement, particularly the brainstem Degree of response to prior intra- and extracranial radiotherapy (identifying dose requirements in relation to expected response) Histopathology and corresponding degree of radiosensitivity/radioresistance RPA-surrogate factors.
Inclusion criteria
Brainstem radiosurgery group (B-RRR): Intrinsic and extrinsic brainstem metastases with or without perilesional edema, with or without fourth ventricle (V4) compression, and the following preexisting conditions:
Patients not candidate for microsurgery, other form of radiotherapy, or systemic (single or concomitant) treatment. Metastases assessed not suitable for SF-GKRS when V10Gy >1 cm3 applying a peripheral prescription dose of 16-18 Gy (single fraction) with prior radiotherapeutic focal impact (including WBRT) or V10Gy >3 cm3 without previous radiotherapy. Dose per fraction assessed by underlying TFVs and structured adaptively in relation to volume kinetics. Karnofsky performance status (KPS) at least 70 and RPA of 1 to 2 when possible. However, exceptions were considered (KPS <70, RPA 3) in cases of CSF-pathway compression (such as V4 compression) requiring acute salvage of the neurological function and/or avoidance of impending neurological death (“compassionate” treatment).
Non-brainstem radiosurgery group (NB-RRR): Metastases with critical location outside brainstem boundaries with or without perifocal edema, with or without CSF pathway compression, with the following preexisting conditions:
Patients not candidate for microsurgery, other form of radiotherapy, or (single/concomitant) systemic treatment targeting the intracranial lesion(s) at hand. Metastases requiring a peripheral dose of 18 Gy or more but not suitable for single dose gamma knife radiosurgery due to large volume (>8-10 cm3). Smaller volumes (<8 cm3) were still assessed as “large” depending on preexistent TFVs (previously described). Dose per fraction assessed by underlying TFVs and structured adaptively in relation to the volume kinetics. KPS at least 70 and RPA of 1 to 2. Exceptions were considered (KPS <70, RPA 3) in cases aiming to avoid further neurological deterioration (compassionate treatment).
Treatment settings
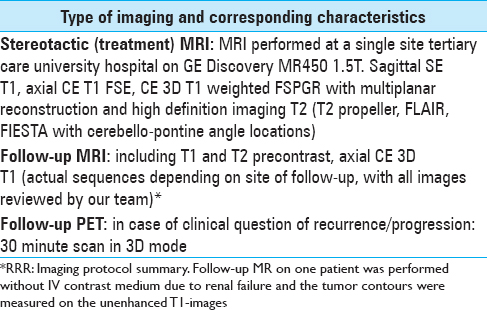
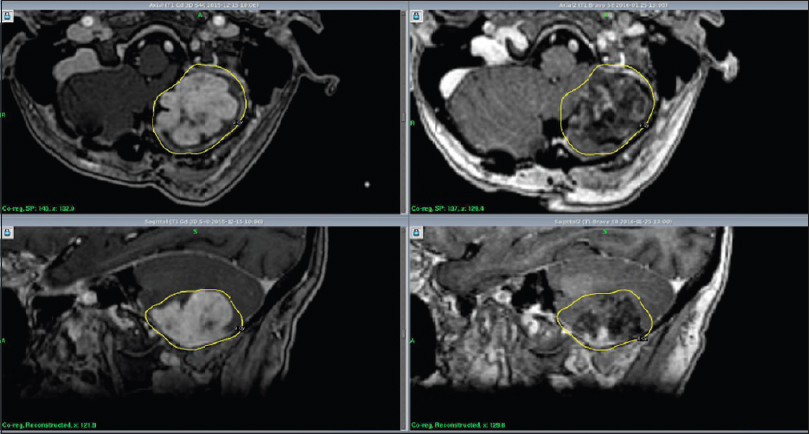
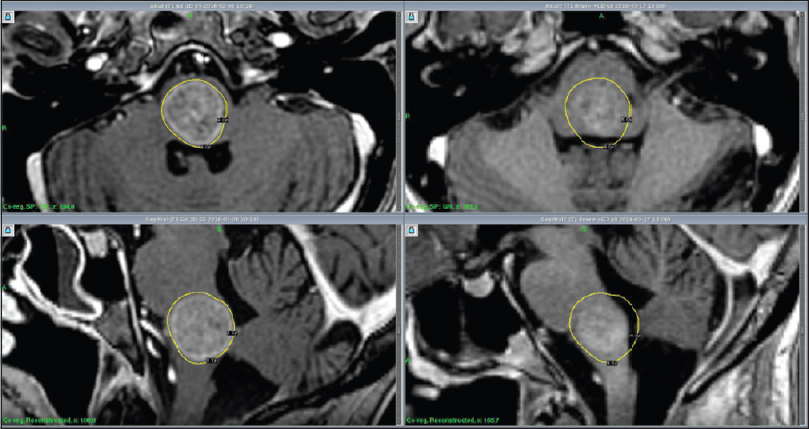
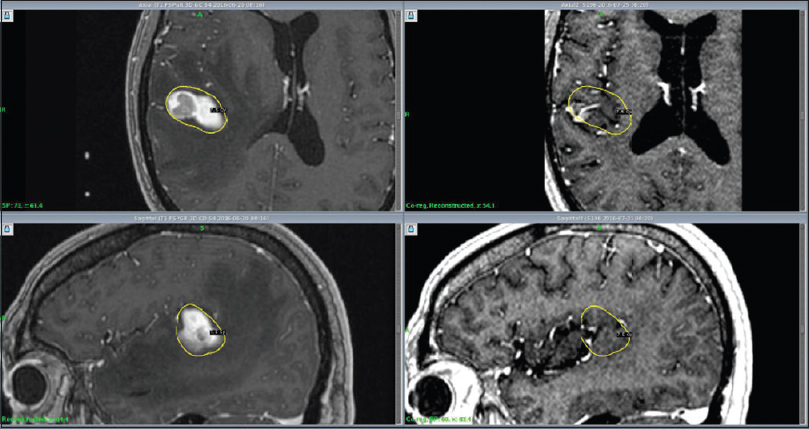
RRR-treatments consisted of three separate GKRS sessions (GKRS 1-3) delivered over a period of 7 days. The Leksell Coordinate Frame G (Elekta AB, Stockholm, Sweden) was mounted under local anesthesia. The three separate stereotactic magnetic resonance imaging (MRI) examinations for gross tumor volume (GTV) delineation included precontrast T1 and T2 weighted sequences and post gadolinium (40 mL IV Dotarem 279.3) 3D T1 weighted sequences on the GE Discovery MR450 1.5T MR [
Thirty-four patients completed the treatment and were included in our analysis [
RESULTS
Clinical outcome
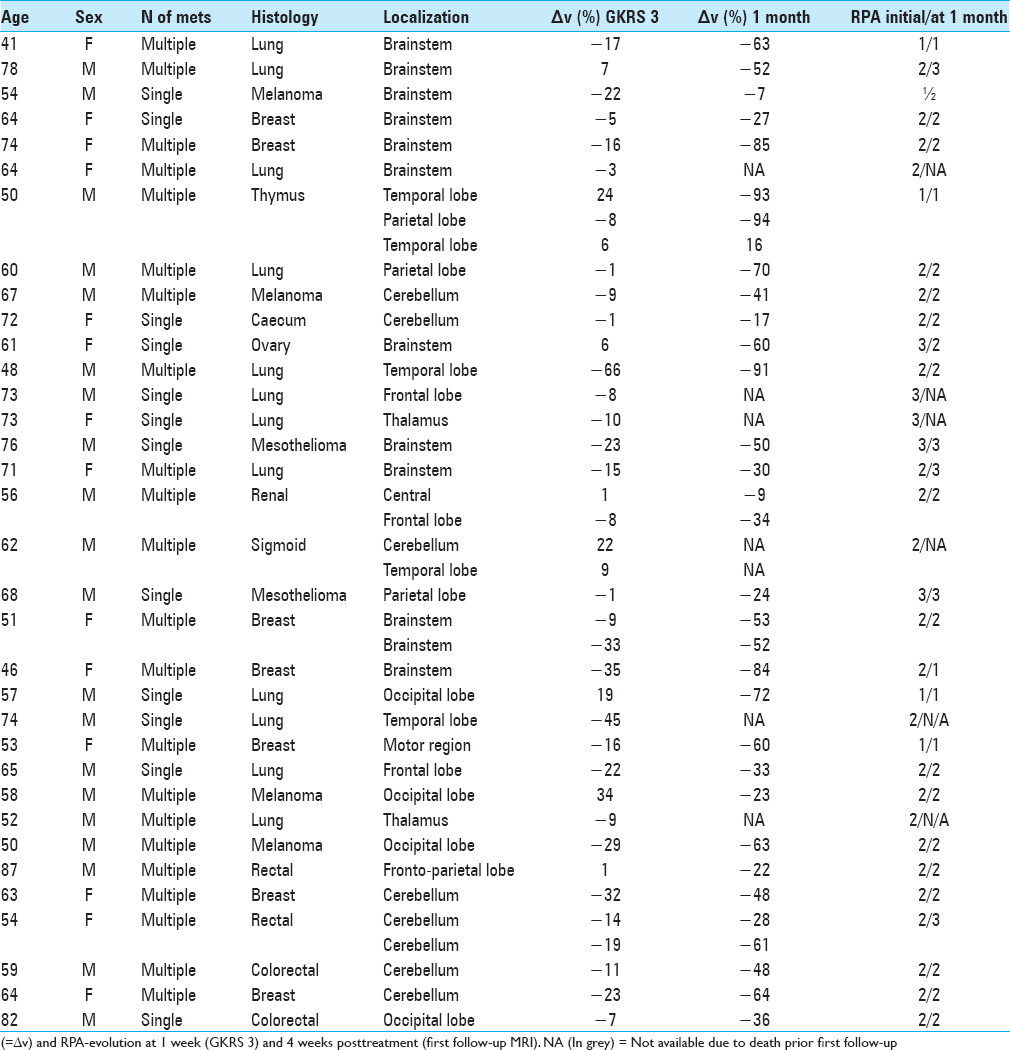
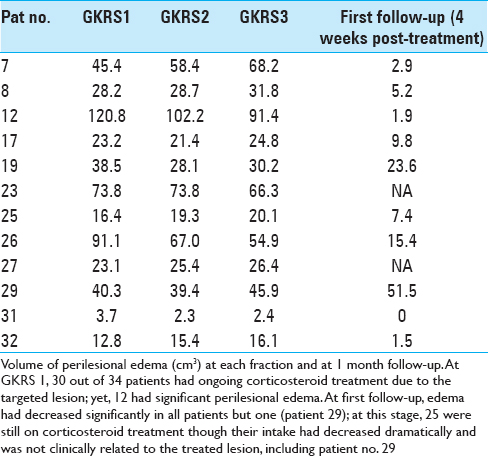
Twenty-eight patients with 33 lesions were able to undergo their first follow-up MRI at 4 weeks posttreatment. The remaining six completed treatment but died prior to their first follow-up due to extracranial complications including a suspected cardiac arrest in one patient and systemic disease progression in the remaining five. No clinical evidence of radiation-related side-effects was reported in the latter group. The median KPS was stable during the course of the treatment. Of the surviving 28 patients evaluated at 4 weeks after treatment completion ( first follow-up MRI), there was a clinical KPS-improvement in eight patients, while 13 patients remained stable. Seven patients experienced KPS-deterioration, although not RRR-related. All patients except three had cortisone treatment at the time of RRR-treatment due to the presence of peritumoral edema. Cortisone treatment was withdrawn prior first follow-up MRI in five patients. The remaining patients had ongoing cortisone at the time of their first follow-up. The RPA of patients who were initially in Class 1 at the time of inclusion (n = 3) remained unchanged at 4 weeks after treatment completion. Among the 25 patients with initial RPA Class 2 (still at the time of inclusion), 15 remained in the same class, 3 patients improved to Class 1, 3 patients deteriorated to Class 3 at first follow-up, while the rest died before the first follow-up. Six patients had an initial RPA class of 3, two of them died of extracranial tumor progression before the first follow-up MRI; among the surviving four patients, two remained in the same class and two patients improved to RPA Class 2 due to KPS-increase [
Radiological outcome
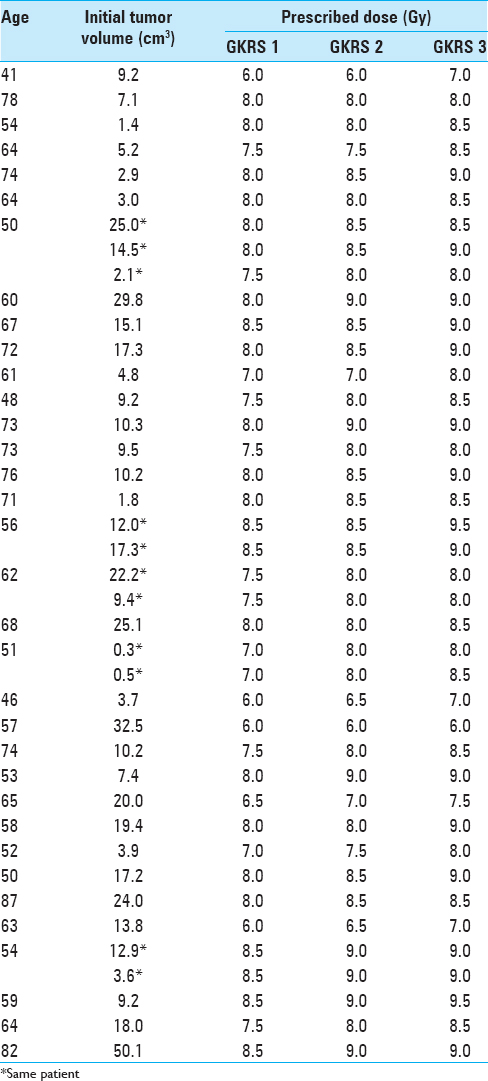
Initial mean tumor volume at GKRS 1 was 12.8 cm3 (range: 0.3-50.1 cm3). Tumor volume reduction during the course of RRR was −6% and −10% at GKRS 2 and GKRS 3, respectively. Mean tumor volume at the first follow-up was available for 33 lesions (28 patients). Average mean tumor volume (at the first follow-up MRI) was 6.0 cm3, ranging between 0.1 and 32 cm3, representing an average reduction of −48% (between GKRS 1 and first follow-up MRI posttreatment) [
DISCUSSION
RRR from a historical perspective
The management of brain metastases in acute and subacute settings remains complex and requires tailored treatment.[
Different groups have reported the application of hypofractionated gamma knife radiosurgery in the management of large brain metastases,[
Eligibility for RRR-treatment is not solely determined by simple, fixed lineal measurements but by the combination of co-existent TFVs and LGP-based GTV estimations. For instance, depending on topographic conditions (such as absence of edema and degree of regional eloquence among others), RRR-treatment thresholds for lesions located outside the brainstem can be set on “standard” volumetric estimates above (GTV >8-10 cm3); yet, smaller metastases (GTV <8 cm3) may be subjected to RRR to avoid SF-GKRS-induced toxicity such as in the case of lesions harbored in highly functional areas.[
Over the years, the RPA classification of brain metastases has been widely used as a prognostic model of treatment response and survival;[
The role of LQ-formalism in RRR-planning
Initial prescription dose at GKRS 1 and MRI-guided peripheral dose augmentation (with subsequent intratumoral escalating dose distribution at GKRS 2 and 3) were conceived by applying LQ-based biologically effective dose (BED) estimates normalized to hypofractionated regimens known to provide local tumor control and limited risk for ARE development. These “reference” schedules ranged between 6-7 Gy × 5 and 7-10Gyx3.[
BAIL: Microenvironmental rationale
Some studies seem to support our line of reasoning;[
As early as 2000, Bussink et al. described how hypoxia decreased while perfusion promptly increased by treating squamous cell carcinoma with 10 Gy.[
Rao et al. reported encouraging results involving acid sphingomyelinase pathways when delivering doses above 8 Gy, subsequently leading to a series of local phenomena such as the activation of tumor endothelial cell apoptosis, disruption of tumor vasculature, and increase in tumor cell death.[
Future strategies: RRR and the role of “immuno-radiosurgery” in anti-cancer treatment
Despite the positive impact of local radiation treatments in terms of local tumor control and survival in general, the mortality rate secondary to overall, whole-body metastatic activity remains an issue. In the same context, RRR may provide an efficient local surgical solution in acute/subacute settings but may not be enough to divert disease progression in the long term, particularly as a monotherapeutic/nonsynergistic approach. However, in the quest for extending the ablative effects of local radiation to distant/peripheral sites, understanding the “bio-mechanical” impact of radiation on immunodynamics remains crucial. In general, the degree and the type of cell death in a tissue-specific fashion is dependent upon variables related to dose and fractionation.[
CONCLUSIONS
In this study, adaptive hypofractionated gamma knife radiosurgery in selected acute/subacute settings (RRR) proved effective in terms of prompt ablation and limited normal tissue toxicity during the week of treatment and 4 weeks after treatment completion ( first follow-up). High performance MRI proved crucial in terms of presurgical diagnostics, treatment planning, and optimal follow-up. We found no strong correlation between RPA classes and (i) survival up to first MRI and (ii) best ablative response during treatment and at first follow-up; this might be due to the limited number of patients included and the short period of time analyzed for the purpose of this study. However, despite the above, we believe intrinsic radiobiological factors such as tumor radiosensitivity and reoxygenation might have played a determinant role in RRR-outcome. Further studies on the subject are required. The potential impact of hypofractionation on crucial immune responses, particularly at vascular checkpoints, is worth developing in the context of RRR-treatments and also warrants further prospective studies. A second complementary paper analyzing the long-term outcome of RRR is being conceptualized; data including; data including local tumor control, ARE-development, overall survival, and clinical status from first to last follow-up will be provided and discussed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank the following team members/colleagues:
-Dr. Heather Martin and Dr. Daniel Martin (Department of Neuroradiology, Karolinska University Hospital, Stockholm, Sweden) for having gone through all relevant MRI studies. Their observations have been of the utmost importance to validate our findings.
References
1. Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved?. Int J Radiat Oncol Biol Phys. 2014. 88: 254-62
2. Bussink J, Kaanders JH, Rijken PF, Raleigh JA, Van der Kogel AJ. Changes in blood perfusion and hypoxia after irradiation of a human squamous cell carcinoma xenograft tumor line. Radiat Res. 2000. 153: 398-404
3. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009. 10: 1037-44
4. Cho YH, Lee JM, Lee D, Park JH, Yoon K, Kim SO. Experiences on two different stereotactic radiosurgery modalities of Gamma Knife and Cyberknife in treating brain metastases. Acta Neurochir. 2015. 157: 2003-9
5. Cohen JV, Kluger HM. Systemic Immunotherapy for the Treatment of Brain Metastases. Front Oncol. 2016. 6: 49-
6. Colliez F, Gallez B, Jordan BF. Assessing Tumor Oxygenation for Predicting Outcome in Radiation Oncology: A Review of Studies Correlating Tumor Hypoxic Status and Outcome in the Preclinical and Clinical Settings. Front Oncol. 2017. 25: 7:10-
7. Crokart N, Jordan BF, Baudelet C, Ansiaux R, Sonveaux P, Grégoire V. Early reoxygenation in tumors after irradiation: Determining factors and consequences for radiotherapy regimens using daily multiple fractions. Int J Radiat Oncol Biol Phys. 2005. 63: 901-10
8. Crokart N, Jordan BF, Baudelet C, Ansiaux R, Sonveaux P, Grégoire V. Early reoxygenation in tumors after irradiation: Determining factors and consequences for radiotherapy regimens using daily multiple fractions. Int J Radiat Oncol Biol Phys. 2005. 63: 901-10
9. Do L, Pezner R, Radany E, Liu A, Staud C, Badie B. Resection followed by stereotactic radiosurgery to resection cavity for intracranial metastases. Int J Radiat Oncol Biol Phys. 2009. 73: 486-91
10. D’Souza NM, Fang P, Logan J, Yang J, Jiang W, Li J. Combining Radiation Therapy with Immune Checkpoint Blockade for Central Nervous System Malignancies. Front Oncol. 2016. 6: 212-
11. Eaton BR, Gebhardt B, Prabhu R, Shu HK, Curran WJ, Crocker I. Hypofractionated radiosurgery for intact or resected brain metastases: Defining the optimal dose and fractionation. Radiat Oncol. 2013. 8: 135-
12. Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: Results and toxicity. Radiother Oncol. 2006. 81: 18-24
13. Fahrig A, Ganslandt O, Lambrecht U, Grabenbauer G, Kleinert G, Sauer R. Hypofractionated stereotactic radiotherapy for brain metastases-results from three different dose concepts. Strahlenther Onkol. 2007. 183: 625-30
14. Formenti SC, Demaria S. Combining Radiotherapy and Cancer Immunotherapy: A Paradigm Shift. J Natl Cancer Inst. 2013. 105: 256-65
15. Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997. 37: 745-51
16. Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol. 2012. 2: 88-
17. Higuchi Y, Serizawa T, Nagano O, Matsuda S, Ono J, Sato M. Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys. 2009. 74: 1543-8
18. Hiroshi K, Hiro S, Yoshiyuki S, Junichi S, Shinei N, Kenichi S. Optimal hypofractionated conformal radiotherapy for large brain metastases in patients with high risk factors: A single-institutional prospective study. Radiat Oncol. 2014. 9: 231-
19. Inoue HK, Sato H, Seto K, Torikai K, Suzuki Y, Saitoh J. Five-fraction Cyberknife radiotherapy for large brain metastases in critical areas: Impact on the surrounding brain volumes circumscribed with a single dose equivalent of 14 Gy (V14) to avoid radiation necrosis. J Radiat Res. 2014. 55: 334-42
20. Inoue HK, Seto K, Nozaki A, Torikai K, Suzuki Y, Saitoh J. Three-fraction CyberKnife radiotherapy for brain metastases in critical areas: Referring to the risk evaluating radiation necrosis and the surrounding brain volumes circumscribed with a single dose equivalence of 14 Gy (V14). J Radiat Res. 2013. 54: 727-35
21. Jaboin JJ, Ferraro DJ, DeWees TA, Rich KM, Chicoine MR, Dowling JL. Survival following gamma knife radiosurgery for brain metastasis from breast cancer. Radiat Oncol. 2013. 8: 131-
22. Janssen MH, Aerts HJ, Kierkels RG, Backes WH, Ollers MC, Buijsen J. Tumor perfusion increases during hypofractionated short-course radiotherapy in rectal cancer: Sequential perfusion-CT findings. Radiother Oncol. 2010. 94: 156-60
23. Joo WK, Hye RP, Jae ML, Jin WK, Hyun-Tai C, Dong GK. Fractionated Stereotactic Gamma Knife Radiosurgery for Large Brain Metastases: A Retrospective, Single Center Study 2016. PLoS One. 2016. 11:
24. Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer. 2016. 4: 51-
25. Kissick M, Campos D, van der Kogel A, Kimple R. On the importance of prompt oxygen changes for hypofractionated radiation treatments. Phys Med Biol. 2013. 58: N279-85
26. Kondziolka D, Shin SM, Brunswick A, Kim I, Silverman JS. The biology of radiosurgery and its clinical applications for brain tumors. Neuro Oncol. 2015. 17: 29-44
27. Kondziolka D, Martin JJ, Flickinger JC, Friedland DM, Brufsky AM, Baar J. Long-term survivors after gamma knife radiosurgery for brain metastases. Cancer. 2005. 104: 2784-91
28. Kurtz G, Zadeh G, Gingras-Hill G, Millar BA, Lap RR, Riere NJ. Salvage radiosurgery for brain metastases: Prognostic factors to consider in patient selection. Int J Radiat Oncol Biol Phys. 2014. 88: 137-42
29. Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y. Therapeutic effects of ablative radiation on local tumor require CD8+T cells: Changing strategies for cancer treatment. Blood. 2009. 114: 589-95
30. Lippitz B, Lindquist C, Paddick I, Peterson D, O’Neill K, Beaney R. Stereotactic radiosurgery in the treatment of brain metastases: The current evidence 2013. Cancer Treat Rev. 2014. 40: 48-59
31. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005. 174: 7516-23
32. Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008. 181: 3099-107
33. McTyre E, Helis CA, Farris M, Wilkins L, Sloan D, Hinson WH. Emerging Indications for Fractionated Gamma Knife Radiosurgery. Neurosurgery. 2017. 80: 210-6
34. Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB. Non-redundant Requirement for CXCR3 Signaling during Tumoricidal T Cell Trafficking across Tumor Vascular Checkpoints. Nat Commun. 2015. 6: 7458-
35. Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F. Single-Fraction Versus Multifraction (3×9 Gy) Stereotactic Radiosurgery for Large (>2 cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int J Radiat Oncol Biol Phys. 2016. 95: 1142-8
36. Navarria P, Pessina F, Cozzi L, Ascolese AM, De Rose F, Fogliata A. Hypo-fractionated stereotactic radiotherapy alone using volumetric modulated arc therapy for patients with single, large brain metastases unsuitable for surgical resection. Radiat Oncol. 2016. 11: 76-
37. Nieder C, Nestle U, Motaref B, Walter K, Niewald M, Schnabel K. Prognostic factors in brain metastases: Should patients be selected for aggressive treatment according to recursive partitioning analysis (RPA) classes?. Int J Radiat Oncol Biol Phys. 2000. 46: 297-302
38. Owonikoko TK, Arbiser J, Zelnak A, Shu HKG, Shim H, Robin AM. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 2014. 11: 203-22
39. Rao SS, Thompson C, Cheng J, Haimovitz-Friedman A, Powell SN, Fuks Z. Axitinib sensitization of high Single Dose Radiotherapy. Radiother Oncol. 2014. 111: 88-93
40. Schlienger M, Nataf F, Huguet F, Pene F, Foulquier JN, Orthuon A. Hypofractionated stereotactic radiotherapy for brain metastases. Cancer Radiother. 2010. 14: 119-27
41. Shibamoto Y, Miyakawa A, Otsuka S, Iwata H. Radiobiology of hypofractionated stereotactic radiotherapy: What are the optimal fractionation schedules?. J Radiat Res. 2016. 57: i76-i82
42. Shibamoto Y, Otsuka S, Iwata H, Sugie C, Ogino H, Tomita N. Radiobiological evaluation of the radiation dose as used in high-precision radiotherapy: Effect of prolonged delivery time and applicability of the linear-quadratic model. J Radiat Res. 2012. 53: 1-9
43. Sinclair G, Samadi A, Martin H, Fagerlund M, Benmakhlouf H, Dodoo E. Adaptive Hypofractionated gamma knife radiosurgery in the acute management of large thymic carcinoma brain metastases. Surg Neurol Int. 2017. 8: 95-
44. Sinclair G, Bartek J, Heather M, Barsoum P, Dodoo E. Adaptive hypofractionated gamma knife radiosurgery for a large brainstem metastasis. Surg Neurol Int. 2016. 7: S130-8
45. Solesvik OV, Rofstad EK, Brustad T. Vascular changes in a human malignant melanoma xenograft following single-dose irradiation. Radiat Res. 1984. 98: 115-28
46. Soliman H, Das S, Larson DA, Sahgal A. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget. 2016. 7: 12318-30
47. Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. 2010. 70: 2697-706
48. Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE. Radiotherapeutic and surgical management for newly diagnosed brain metastasis (es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012. 2: 210-25
49. Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015. 33: 445-74
50. Yomo S, Hayashi M. A minimally invasive treatment option for large metastatic brain tumors: Long-term results of two-session Gamma Knife stereotactic radiosurgery. Radiat Oncol. 2014. 9: 132-
51. Zada G, Yu C, Pagnini PG, Khalessi AA, Zelman V, Apuzzo ML. Early decreased tumor volume following fractionated Gamma Knife Radiosurgery for metastatic melanoma and the role of “adaptive radiosurgery”: Case report. Neurosurgery. 2010. 67: E512-3