- Department of Neurosurgery, Cairo University, Cairo, Egypt.
Correspondence Address:
Mohamed Eltoukhy, Department of Neurosurgery, Cairo University, Cairo, Egypt.
DOI:10.25259/SNI_1072_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohamed Eltoukhy, Karim Eldabaa, Mohamed Ahmed Eissa. The effect of epidural steroid injection during surgery on surgical site infections following lumbar fusion. 29-Sep-2023;14:348
How to cite this URL: Mohamed Eltoukhy, Karim Eldabaa, Mohamed Ahmed Eissa. The effect of epidural steroid injection during surgery on surgical site infections following lumbar fusion. 29-Sep-2023;14:348. Available from: https://surgicalneurologyint.com/surgicalint-articles/12573/
Abstract
Background: Intraoperative epidural steroid injections (ESIs) have been suggested to limit pain following lumbar fusions. However, the frequency of resultant surgical site infections has not been fully investigated.
Methods: We retrospectively followed two groups of patients; 23 patients were the control group, while the other 23 patients received, in addition to the spinal fusions, intraoperative ESI.
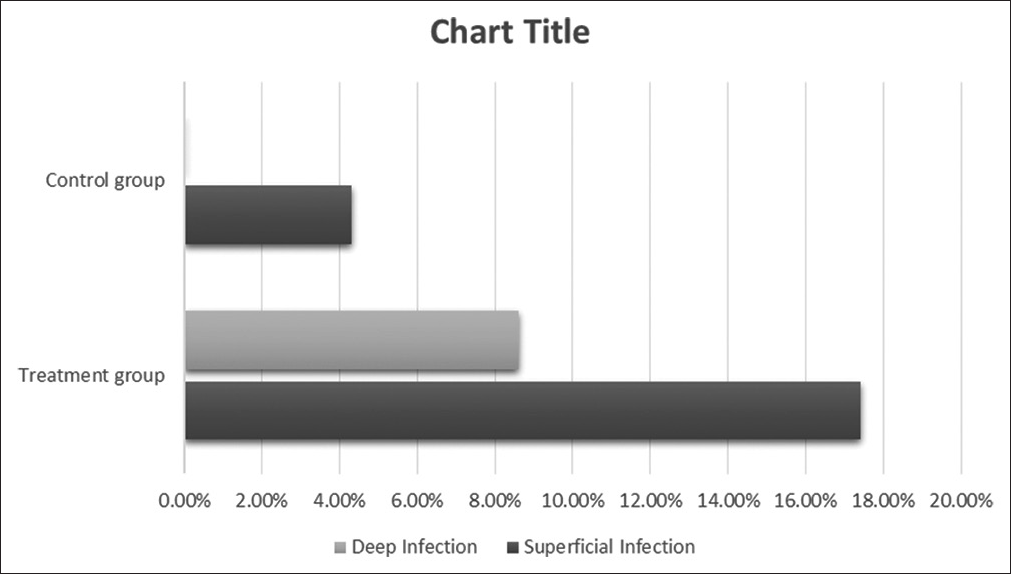
Results: Patients in the latter ESI/fusion treatment group had significantly increased rates of superficial and deep infections (i.e., superficial infections 17.4% and 4.3% deep infections) versus control patients (i.e., 8.6% superficial and 0% deep) undergoing fusions alone.
Conclusion: We observed an increased risk of postoperative surgical site infections among patients who underwent intraoperative ESI in addition to their lumbar fusions.
Keywords: Epidural steroid injection, Lumbar fusion, Surgical site infection
INTRODUCTION
Intraoperative epidural steroid injection (ESI) has been suggested as a solution to the 40% incidence of pain reported following lumbar fusions.[
MATERIALS AND METHODS
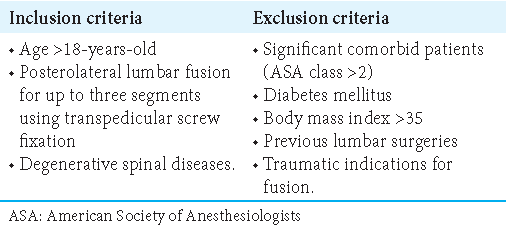
From 2020 to 2022, we retrospectively compared the incidence of superficial versus deep infections for 23 patients undergoing ESI (7–14 mg of Betamethasone injected under direct vision) during instrumented lumbar fusions (i.e., 3-segment posterolateral lumbar fusions using transpedicular screw fixation) versus 23 control patients having lumbar fusions alone. Inclusion and exclusion criteria are outlined in
RESULTS
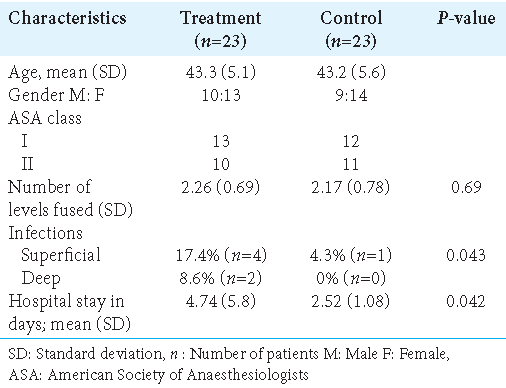
Both groups had comparable demographics and comorbidities [
Treatment of superficial and deep postoperative infections
The total rate of infection in the treatment group was 17.4% (4 patients) for superficial infections and 8.6% (2 patients) for deep infections versus only 4.3% (1 patient) for superficial infections in the control (i.e., no ESI) group [
DISCUSSION
Our study (2020–2022) included 23 patients who received ESI during instrumented fusions versus 23 patients control patients not treated with ESI. We found statistically significant increases in early infections among patients receiving ESI during their fusions (i.e., 4 of 23 superficial infections and 2 of 23 deep infections) versus just one superficial infection for 23 patients undergoing fusions alone. This resulted in a longer average LOS of 4.74 days for the 23 ESI/fusion patients versus 2.52 days for the 23 control patients. Kreitz et al. also found the fusion group showed an increased risk of infection if an ESI was administered before fusion surgery versus those without (2.68% vs. 1.69%).[
CONCLUSION
The literature showed that ESI within 30 days to 6 months before spinal surgery increases the risk of both superficial and deep postoperative spinal infections. Further, this study indicated that the additional intraoperative administration of ESI increased the risk of both superficial (i.e., 4 of 23) and deep (i.e., 2 of 23) infections after spine fusion surgery versus just one of 23 patients undergoing lumbar surgery/fusions without ESI.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Donnally CJ, Rush AJ, Rivera S, Vakharia RM, Vakharia AM, Massel DH. An epidural steroid injection in the 6 months preceding a lumbar decompression without fusion predisposes patients to post-operative infections. J Spine Surg. 2018. 4: 529-33
2. Kreitz TM, Mangan J, Schroeder GD, Kepler CK, Kurd MF, Radcliff KE. Do preoperative epidural steroid injections increase the risk of infection after lumbar spine surgery?. Spine (Phila Pa 1976). 2021. 46: E197-202
3. Li Z, Liu P, Zhang C, Xu G, Zhang Y, Chang Y. Incidence, prevalence, and analysis of risk factors for surgical site infection after lumbar fusion surgery:≥ 2-year follow-up retrospective study. World Neurosurg. 2019. 131: e460-7
4. Malhotra AK, Wilson JR. Topical epidural steroids after lumbar spine surgery: Do the benefits observed after microdiscectomy extend to lumbar fusion?. J Neurosurg Spine. 2022. 37: 473-5
5. Ranguis SC, Li D, Webster AC. Perioperative epidural steroids for lumbar spine surgery in degenerative spinal disease. A review. J Neurosurg Spine. 2010. 13: 745-57
6. Tavanaei R, Ahmadi P, Malekipour B, Biazar BH, Keikhaee M, Yazdani KO. Effects of local intraoperative epidural use of triamcinolone acetonide-soaked Gelfoam on postoperative outcomes in patients undergoing posterolateral lumbar spinal fusion surgery: A randomized, placebo-controlled, double-blind trial. J Neurosurg Spine. 2022. 37: 476-84