Unique Case Observations
Case Report
- Department of Neurosurgery, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy.
- Department of Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy.
Correspondence Address:
Ignazio Gaspare Vetrano
Department of Neurosurgery, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy.
DOI:10.25259/SNI_296_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ignazio Gaspare Vetrano1, Francesco Acerbi1, Gianluca Marucci2, Vittoria Nazzi1. The effect of ozone injection within a common peroneal nerve schwannoma: A mistreatment due to a misdiagnosis. 04-Dec-2020;11:413
How to cite this URL: Ignazio Gaspare Vetrano1, Francesco Acerbi1, Gianluca Marucci2, Vittoria Nazzi1. The effect of ozone injection within a common peroneal nerve schwannoma: A mistreatment due to a misdiagnosis. 04-Dec-2020;11:413. Available from: https://surgicalneurologyint.com/surgicalint-articles/the-effect-of-ozone-injection-within-a-common-peroneal-nerve-schwannoma-a-mistreatment-due-to-a-misdiagnosis/
Abstract
Background: Peripheral schwannomas can be misdiagnosed or mistreated as they can mimic other subcutaneous lesions, leading to wrong diagnosis and, therefore, to improper treatment.
Case Description: A 23-years-old male presented a painful growing nodule at the left popliteal fossa, with distally irradiated pain. A first magnetic resonance imaging depicted a heterogeneous lesion between common peroneal and sural nerves but, surprisingly, the patient was submitted to perilesional injection of ozone-oxygen mixture, causing the onset of intense neuropathic pain. A second MRI showed a morphological change of tumor characteristics. He finally underwent surgery but, intraoperatively, inter-fascicular fibrous adherences were noticed, making the tumor removal more difficult and riskier. The histopathological diagnosis was of schwannoma with areas of foreign body reaction.
Conclusion: The injection of ozone or other substances within a subcutaneous swelling should be avoided, before a complete imaging assessment; because of such swelling could be a peripheral nerve schwannoma. The correct assessment of a lesion of the limbs determining radiating pain should be carefully demanded to a thorough history, clinical examination, and appropriate imaging technique. To avoid incorrect management, the treatment of such tumors should be performed in the first place by dedicated equips with proven expertise in this field.
Keywords: Common peroneal nerve, Foreign body reaction, Nerve tumors, Oxygen-ozone therapy, Schwannoma
INTRODUCTION
Schwannomas, the most common peripheral nerve sheath tumors (PNST), are well-circumscribed lesions within peripheral nerves.[
During the past years, oxygen-ozone therapy (OT) has been employed in several pathologies, mainly in painful syndromes,[
We report a case of a young male harboring a lower limb schwannoma, previously treated with percutaneous intralesional OT, causing morphological, and architectural tumor’s alteration. The subsequent the formation of strong adhesions between the neoplasm and the originating nerve finally led to a more dangerous surgical procedure, resulting in transient slight neurological worsening.
CASE PRESENTATION
In May 2019, a 23-year-old male was evaluated at our institution for a 1-year history of a painful subcutaneous nodule at the left popliteal fossa. The pain, described as an “electric shock.” was also irradiated to the lateral surface of the leg. He then underwent ultrasonography, with a first diagnosis of subcutaneous cyst; subsequently, he was submitted in another institution, under local anesthesia, to an attempt for surgical removal by a dermatology specialist; however, the procedure was aborted due to the onset of acute pain. A first MRI [
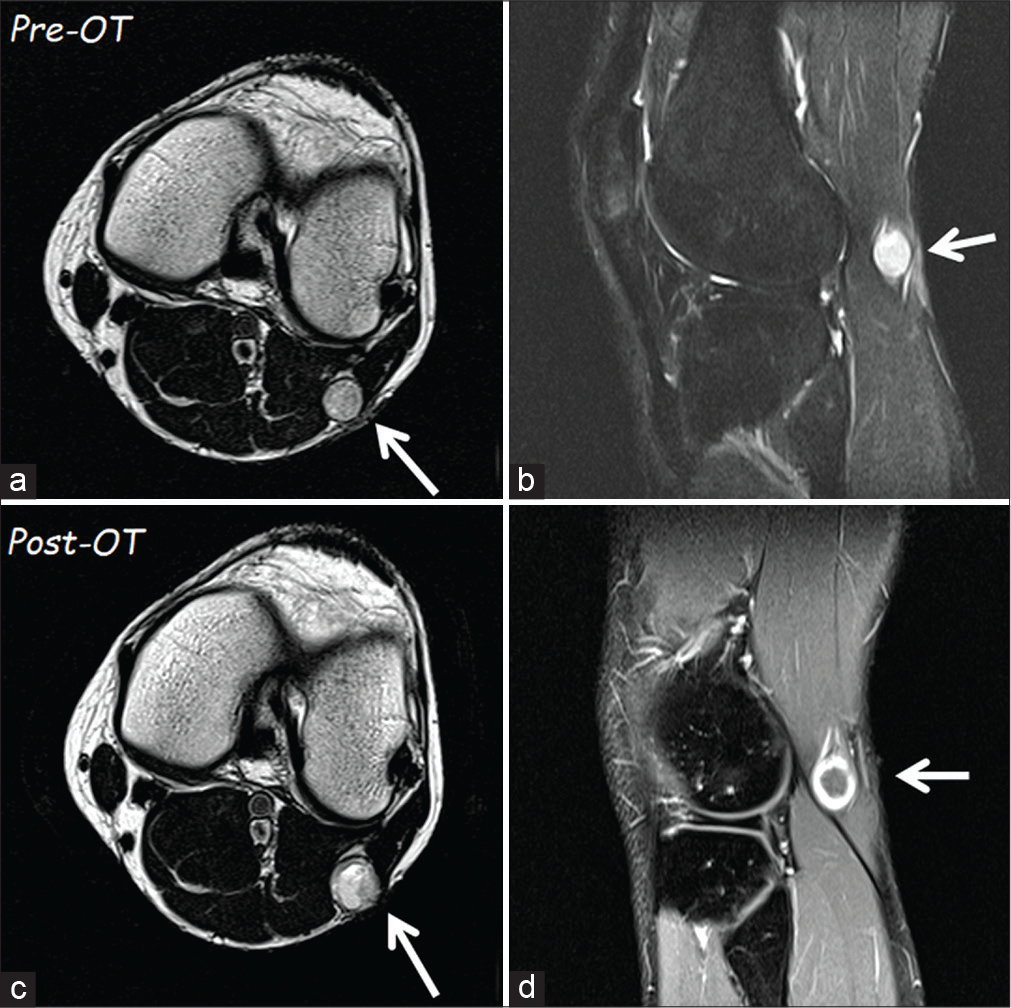
Figure 1:
The first magnetic resonance imaging (MRI) of November 2018 (a – T1 axial and b – sagittal scans) showed a homogeneous lesion (arrow) of 12 × 13 mm at the fibula’s head level. The second MRI in axial (c) scan, performed after oxygen-ozone therapy, documented a slight increase of the tumor size. It is evident a change in tumor intensity, which had become inhomogeneous, and with a rim of contrast enhancement (d).
Surgical treatment
The patient finally underwent surgery under microscopic view, with intraoperative neurophysiological monitoring. Continuous free-running and stimulus-triggered EMG was used to identify functioning nerves and to localize the safest entry point inside the tumor capsule. Sodium fluorescein 1 mg/kg was intravenously injected by the anesthesiologist, on completion of the induction, as previously described.[
A slight curvilinear incision at the level of the lateral and inferior part of the popliteal fossa was performed and, after subcutaneous dissection, we identified an encapsulated lesion between the common peroneal and sural nerves. Since the dissection of the pseudocapsule, a fibrous reaction was noticed, which made it slightly more challenging to identify the tumor capsule and a silent, safe zone without positive electrophysiological responses. Several strong adherences were present, and the consistency of the neoplasm was harder than usual. These factors determined the impossibility to remove the tumor en block; it was instead removed through a gentle piecemeal resection [
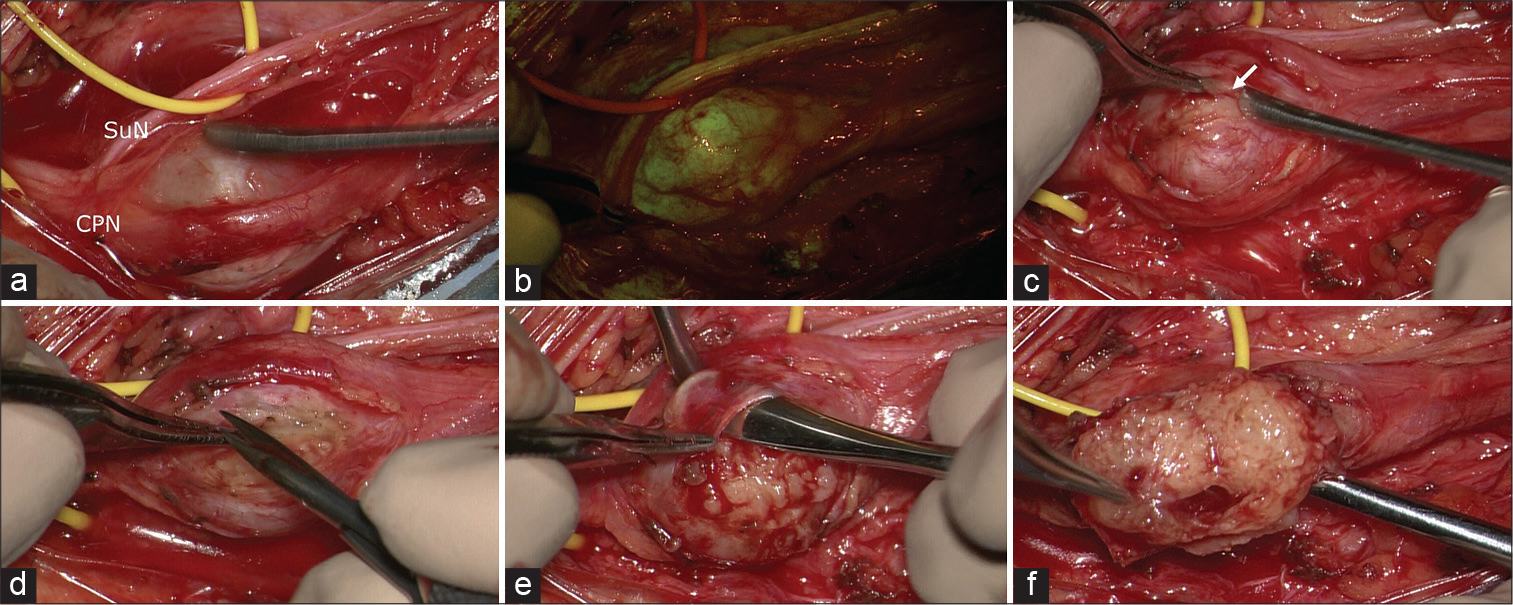
Figure 2:
Intraoperative photographs under microscopic view: in (a), the schwannoma is visible between common peroneal nerve and sural nerve. After the YELLOW560 filter activation, the tumor showed a fluorescein uptake more intense than the surrounding nerves (b). The capsule (arrow in c) appeared extremely thick and adherent to the tumor and the originating nerve, requiring multiple manipulations for intraneural dissection and schwannoma removal (d-f).
Histopathological analysis
Histological examination [
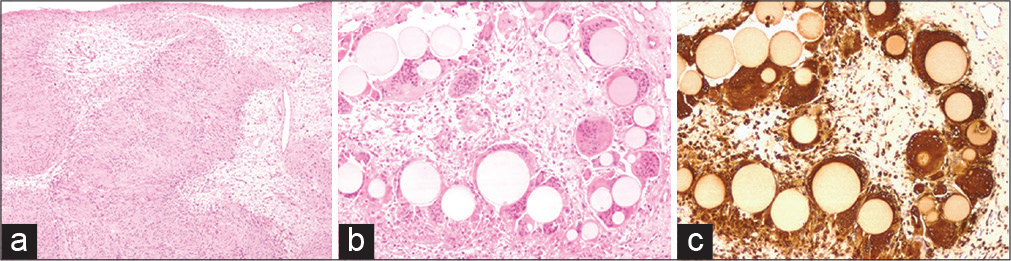
Figure 3:
The histological examination revealed (a) a nerve sheath tumor constituted by well-differentiated Schwann cells, characterized by nuclear palisades consistent with Verocay bodies (H and E, ×40). The microscopic study at ×100 highlighted foci of foreign body reaction (H and E in b) with uniformly sized, grey-yellowish microspheres phagocytized by multinucleated giant cells (immunostained with CD68 in c).
Postoperative outcome
After surgery, the patient presented worsening of the left foot extension, while local and irradiated pain disappeared. He was then referred to physical therapy: 3-months after surgery, the motor deficit was almost completely regressed. At 12-months follow-up examination, the motor function had completely recovered. The last nerve ultrasonography depicted a discreetly preserved structure, without focal section enlargement.
DISCUSSION
Surgery is the gold-standard treatment in peripheral nerve schwannomas: after capsule and pseudocapsule incision, it is possible to remove the tumor safely, following the right surgical steps.[
Despite the lack of specific literature about the delay in the diagnosis of the lower limb schwannomas, PNST can sometimes be confused with other diseases, as lipomas.[
OT has been employed for the treatment of several conditions, in particular loco-regional pain syndromes, low back pain, arteriosclerosis, and arthritis.[
In our case, the schwannoma was harder and with several adherences within the fascicles, leading to maneuvers that are more complex and, potentially, more dangerous, to separate the tumor from the intact fascicles. The need for such maneuvers, despite the intraoperative neurophysiological monitoring, finally led to the transient neurological worsening.
CONCLUSION
The injection of ozone or other substances within a subcutaneous swelling should be avoided, before a complete imaging evaluation; because of such swelling could be a peripheral nerve schwannoma. The management of tumors should be demanded to neurosurgeons with expertise in this field to avoid a wrong treatment.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated during the current study.
Declaration of patient consent
The study protocol was in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration of 1970 as amended in 2000.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Apuzzo D, Giotti C, Pasqualetti P, Ferrazza P, Soldati P, Zucco GM. An observational retrospective/horizontal study to compare oxygen-ozone therapy and/or global postural reeducation in complicated chronic low back pain. Funct Neurol. 2014. 29: 31-9
2. Elvis AM, Ekta JS. Ozone therapy: A clinical review. J Nat Sci Biol Med. 2011. 2: 66-70
3. Fass G, Hossey D, Nyst M, Smets D, Saligheh EN, Duttmann R. Benign retroperitoneal schwannoma presenting as colitis: A case report. World J Gastroenterol. 2007. 13: 5521-4
4. Guha D, Davidson B, Nadi M, Alotaibi NM, Fehlings MG, Gentili F. Management of peripheral nerve sheath tumors: 17 years of experience at Toronto Western Hospital. J Neurosurg. 2018. 128: 1226-34
5. Gürkan G, Sayin M, Kizmazoglu C, Erdogan MA, Yigitturk G, Yilmaz HE. Evaluation of the neuroprotective effects of ozone in an experimental spine injury model. J Neurosurg Spine. 2020. p. 1-9
6. Kara Ö Kara M. Lipolysis of a painful lipoma with ozone: The role of ultrasound in the diagnosis and quantification of the treatment. Med Gas Res. 2019. 9: 168
7. Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University health sciences center. J Neurosurg. 2005. 102: 246-55
8. Muto M, Giurazza F, Silva RP, Guarnieri G. Rational approach, technique and selection criteria treating lumbar disk herniations by oxygen-ozone therapy. Interv Neuroradiol. 2016. 22: 736-40
9. Ongley D, Shipton E. Pain images: Hip pain-wrong diagnosis, wrong operation. Pain Med. 2010. 11: 942-5
10. Resnick JM, Fanning CV, Caraway NP, Varma DG, Johnson M. Percutaneous needle biopsy diagnosis of benign neurogenic neoplasms. Diagn Cytopathol. 1997. 16: 17-25
11. Rilling S, Viebahn R.editors. The Use of Ozone in Medicine. Heidelberg: KF Haug Publishers; 1987. p.
12. Rowen RJ, Robins H. Ozone therapy for complex regional pain syndrome: Review and case report. Curr Pain Headache Rep. 2019. 23: 41
13. Russell SM. Preserve the nerve: Microsurgical resection of peripheral nerve sheath tumors. Neurosurgery. 2007. 61: 113-7
14. Singh V, Kapoor R. Atypical presentations of benign retroperitoneal schwannoma: Report of three cases with review of literature. Int Urol Nephrol. 2005. 37: 547-9
15. Smith N, Wilson A, Gandhi J, Vatsia S, Khan S. Ozone therapy: An overview of pharmacodynamics, current research, and clinical utility. Med Gas Res. 2017. 7: 212-9
16. Stone JJ, Spinner RJ. Go for the gold: A plane and simple technique for resecting Benign peripheral nerve sheath tumors. Oper Neurosurg (Hagerstown). 2020. 18: 60-8
17. Tiel R, Kline D. Peripheral nerve tumors: Surgical principles, approaches, and techniques. Neurosurg Clin N Am. 2004. 15: 167-75
18. Vanni D, Galzio R, Kazakova A, Pantalone A, Sparvieri A, Salini V. Intraforaminal ozone therapy and particular side effects: Preliminary results and early warning. Acta Neurochir (Wien). 2016. 158: 491-6
19. Vetrano IG, Acerbi F, Falco J, Devigili G, Rinaldo S, Messina G. Fluorescein-guided removal of peripheral nerve sheath tumors: A preliminary analysis of 20 cases. J Neurosurg. 2019. p. 1-10
20. Vetrano IG, Lucarella F, Dalolio M, di Cristofori A, Nataloni IF, Tiberio F. The importance of predicting factors in the surgical outcome of peripheral nerve sheath tumors. J Neurol Surg A Cent Eur Neurosurg. 2014. 75: 104-9
21. Vetrano IG, Saletti V, Nazzi V. Fluorescein-guided resection of plexiform neurofibromas: How i do it. Acta Neurochir (Wien). 2019. 161: 2141-5
22. Zaidman CM, Seelig MJ, Baker JC, Mackinnon SE, Pestronk A. Detection of peripheral nerve pathology: Comparison of ultrasound and MRI. Neurology. 2013. 80: 1634-40
23. Zou F, Dai M, Zhang B, Nie T. Misdiagnosis of a giant intrapelvic schwannoma: A case report. Oncol Lett. 2013. 6: 1646-8