- Department of Neurological Surgery, University of Miami MILLER School of Medicine Jacson Memorial Hospital, Miami, Florida, United States,
- Neurosciences Center, King Faisal Specialist Hospital and Research Center,
- Department of Pathology and Laboratory Medicine, King Faisal specialist Hospital and Research Center, Riyadh, Saudi Arabia.
Correspondence Address:
Imad N Kanaan, Neurosciences Center, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_664_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Turki Elarjani1, Meshari Rashed Alhuthayl2, Hindi Alhindi3, Imad N Kanaan2. The effect of radiation therapy and chemotherapy on malignant craniopharyngioma: A review. 25-Oct-2021;12:539
How to cite this URL: Turki Elarjani1, Meshari Rashed Alhuthayl2, Hindi Alhindi3, Imad N Kanaan2. The effect of radiation therapy and chemotherapy on malignant craniopharyngioma: A review. 25-Oct-2021;12:539. Available from: https://surgicalneurologyint.com/surgicalint-articles/11198/
Abstract
Background: Malignant craniopharyngioma is a rare tumor with few published case reports. It can form de novo or transform from a benign variant and is associated with a dismal survival rate. We reviewed the literature for all published cases and studied the effect of radiation on the rate of malignant transformation. We analyzed the effect of chemotherapy on survival.
Methods: We used various search engines to locate literature from 1980 onward and identified 31 case reports, one of which was excluded. Statistical analysis using the SAS software was conducted, and a significant value was identified if P
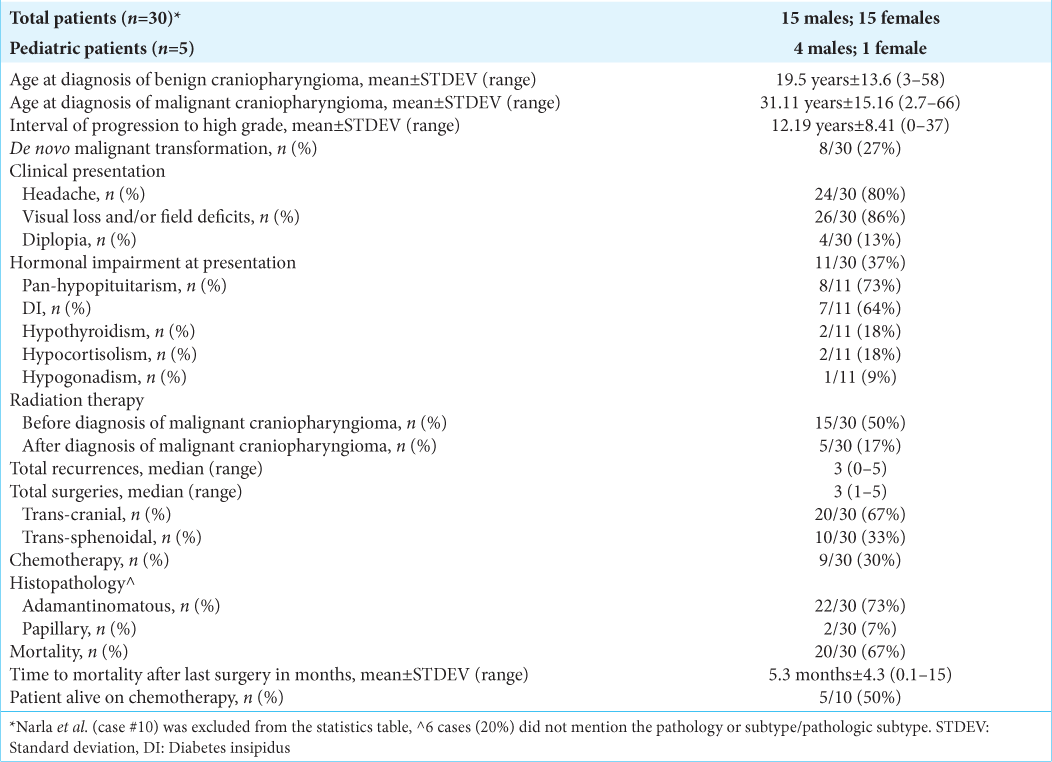
Results: There was equal distribution among male and female patients. The average age at malignant diagnosis is 31.11 years (±15.16) and 12.19 years (±8.41) for the average interval of benign tumor progression to malignancy. The most common clinical presentation was visual loss and/or field deficits in 26/30 patients (86%). Almost 11/30 patients (37%) had endocrinological deficits, with panhypopituitarism as the most common in 8/11 patients (73%). Fifteen patients received radiation before malignant transformation (47%) and demonstrated no effect on malignant transformation (P = 0.379). Gross total resection was achieved in 2/30 patients. The average time to mortality postoperatively is 5.3 months ± 4.3. Ten patients received chemotherapy, and five were alive at last follow-up (P = 0.115).
Conclusion: Malignant craniopharyngioma carries a dismal prognosis with no apparent benefits of radiation therapy and chemotherapy on survival.
Keywords: Craniopharyngioma, Pituitary, Recurrent
INTRODUCTION
Craniopharyngioma is an uncommon, benign epithelial tumor derived from buccal mucosa rests or Rathke’s pouch remnants.[
Rarely, craniopharyngiomas present as a de novo malignant tumor or a transformation from an already existent benign tumor. The few case reports published in the literature demonstrated high morbidity and mortality rates.[
MATERIALS AND METHODS
A literature review in Medline, Web of Science, and Scopus was used to locate existing case reports. The search included the following terms: “craniopharyngioma” AND “malignancy” OR “high-grade” OR “malignant transformation.” All articles written in English and published in 1980 and beyond were included in the study, while non-English articles and articles published before 1980 were excluded from the study. Articles published before 1980 had substantially different diagnostic modalities and treatment approaches not applicable in the current era. The databases were last queried in May 2020. One article did not mention the outcome of their patient, so it was excluded from the statistical summary data.
We examined different variables, such as the incidence in males and females, age at diagnosis of benign craniopharyngioma, age at diagnosis of malignant craniopharyngioma, the time interval for malignant transformation, clinical presentation, hormonal deficits, radiation therapy before and after malignant transformation, lesion recurrence (including benign craniopharyngioma), total surgeries, chemotherapy administration, histopathology, and outcome. De novo malignant craniopharyngioma is defined as a craniopharyngioma that was initially diagnosed as malignant in histological studies. Secondary malignant craniopharyngioma is defined as a craniopharyngioma that was initially diagnosed as benign and then underwent malignant transformation overtime. Headache was a subjective complaint by the patients in the case reports, while visual loss and/or field and diplopia were subjective complaints and objective findings on physical examination. Most articles did not mention the instrument used in the transsphenoidal approach (endoscopic vs. microscopic).
CASE ILLUSTRATION
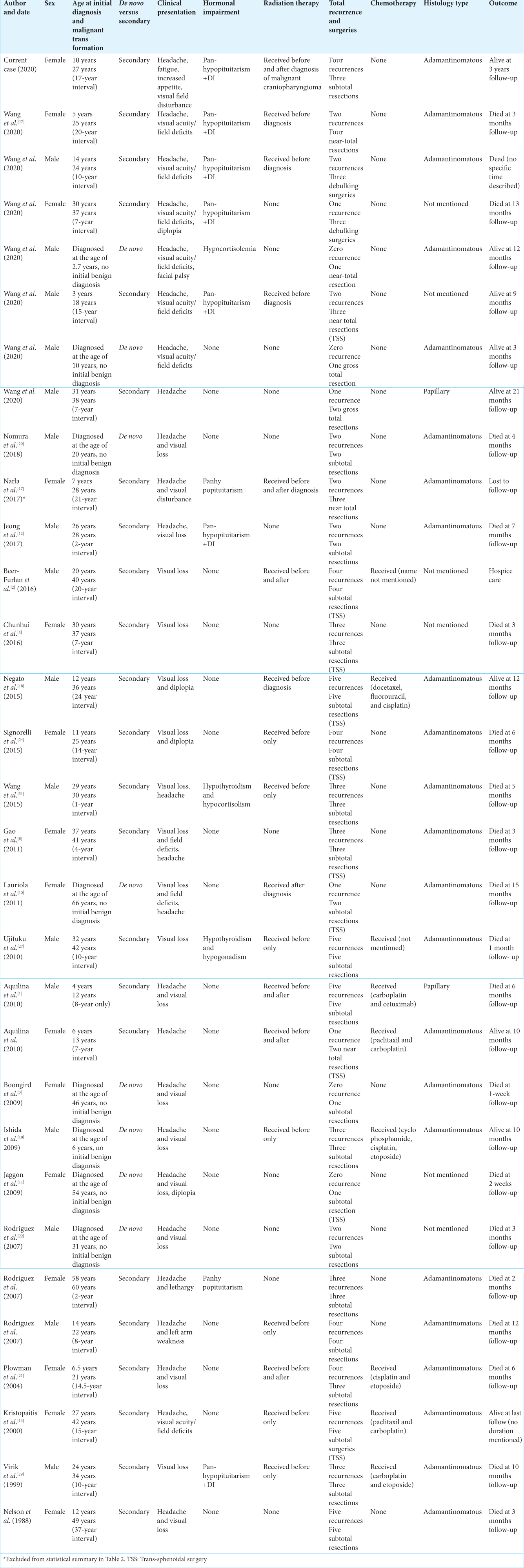
This is a 27-year-old female who was diagnosed with benign craniopharyngioma in 1999 and had underwent a right pterional craniotomy with subtotal resection (STR), followed by 50.4 Gy in 28 fractions radiation. She received hormonal replacement due to panhypopituitarism, and in 2017, the patient presented with progressive fatigue and thirst and polyuria and bitemporal hemianopsia. Magnetic resonance imaging (MRI) and computed tomography (CT) demonstrate a suprasellar cystic lesion [
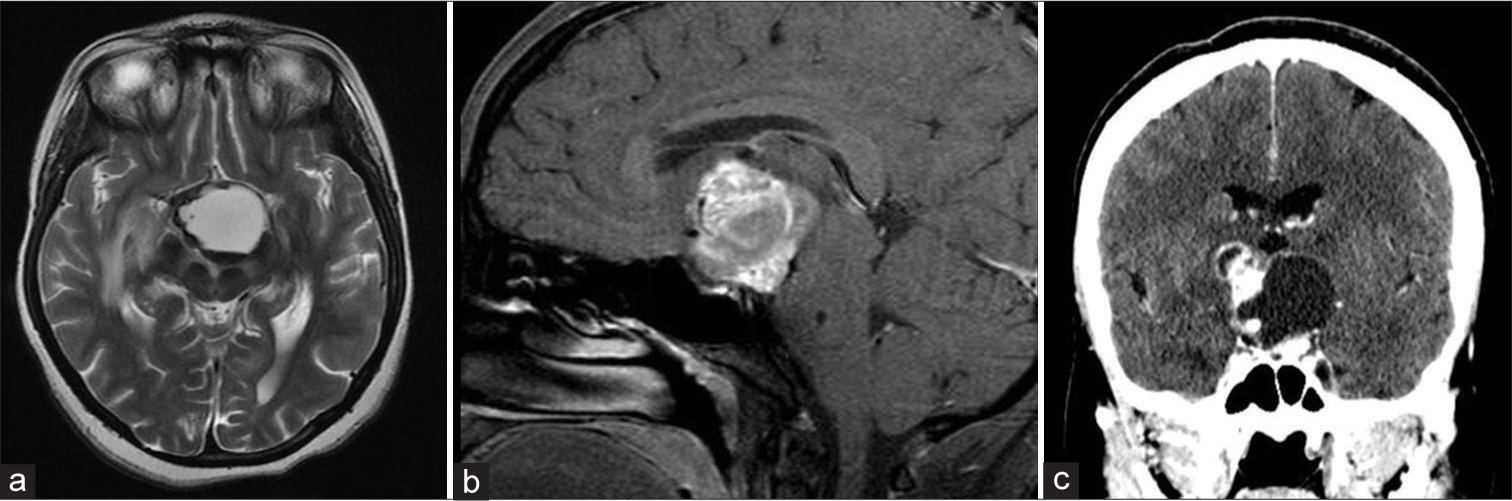
Figure 1:
Axial magnetic resonance imaging (MRI) T2 (a) sagittal MRI T1 with contrast (b) and computed tomography (CT) coronal view (c) revealing a large cystic lesion with solid component occupying the suprasellar region exerting mass effect on the surrounding neurovascular structure CT image is showing the calcification component of the lesion.
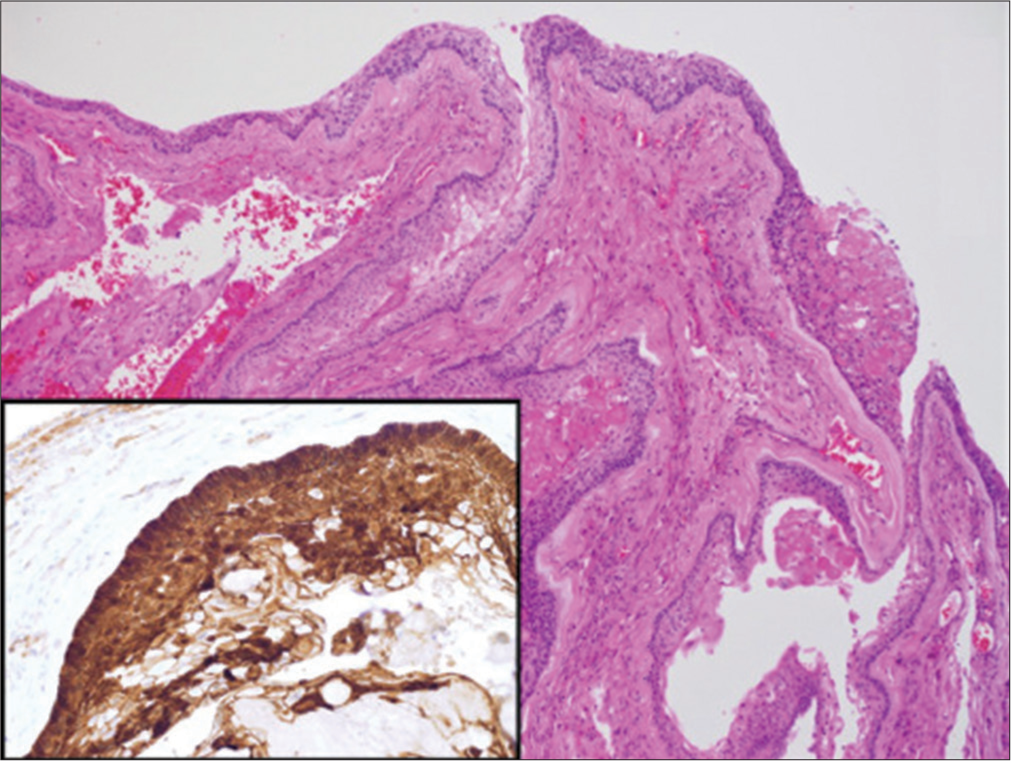
The patient underwent a right pterional craniotomy and reresection of the lesion and her clinical symptoms improved with persistent panhypopituitarism. Histopathology revealed malignant features of the lesion [
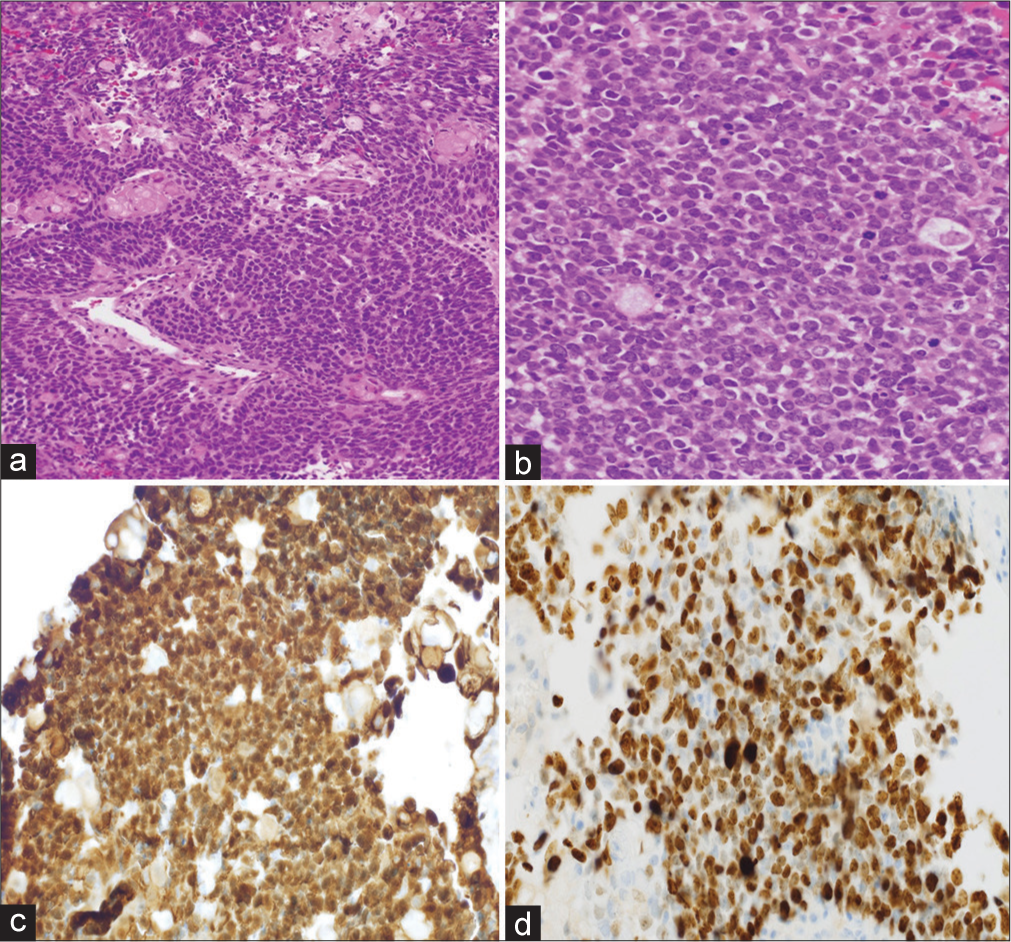
Figure 3:
Low magnification view of the malignant component showing the highly cellular epithelial sheets (a) and high magnification (b) shows the high nucleus-cytoplasm ratio and nuclear atypia and scattered mitotic figures. Note the nuclear expression of beta-catenin (c) and the high MIB-1 proliferation index (d) (a: H&E original magnification ×40 and b: H&E original magnification ×400 and c: beta-catenin immunohistochemistry original magnification ×200 and d: Ki-67 immunohistochemistry original magnification ×200).
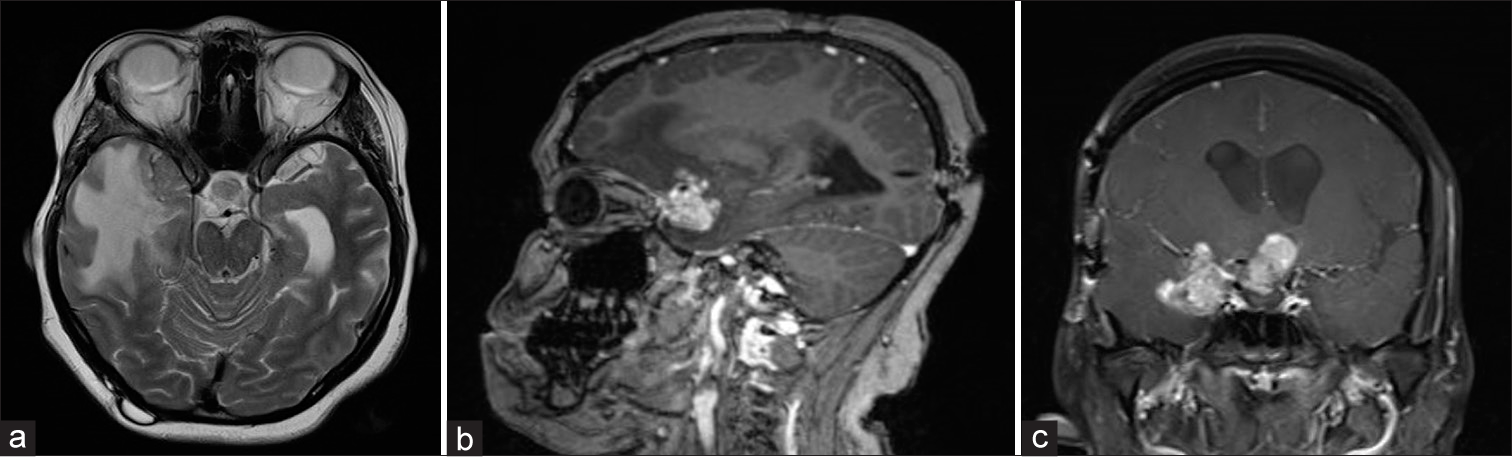
Figure 4:
Axial magnetic resonance imaging (MRI) T2 (a) and sagittal MRI T1 with contrast (b) and coronal MRI T1 with contrast (c) demonstrating a lesion recurrence in the suprasellar region with new extension to the right middle cranial fossa and surrounding right temporal vasogenic edema is noted. Occipital VP shunt is visible on the sagittal image.
Statistical analysis
We used the Statistical Analysis System (SAS) software (SAS version 9.4, SAS Institute Inc., Cary, NC, USA) for statistical analysis. Categorical variables are presented as frequency and percentage and continuous variables are presented as mean (standard deviation) for normal distribution and median (interquartile range) for non-normal distribution of the data. We performed an independent t-test analysis for normally distributed quantitative variables and a Mann–Whitney U-test for non-normally distributed quantitative variables. A Chi-square analysis was used for qualitative variables, and Fisher’s exact test was used for variables with a sample number below 5.
RESULTS
We identified 31 patients satisfying the inclusion criteria published between 1988 and 2020 [
Figure 5:
Repeat axial magnetic resonance imaging (MRI) with contrast in 2020 (a) and sagittal MRI T2 (b) and coronal MRI T1 with contrast (c) showing significant interval growth of the suprasellar mass occupying the anterior third ventricle and the interpeduncular cistern with evident mass effect on the surrounding neurovascular structures.
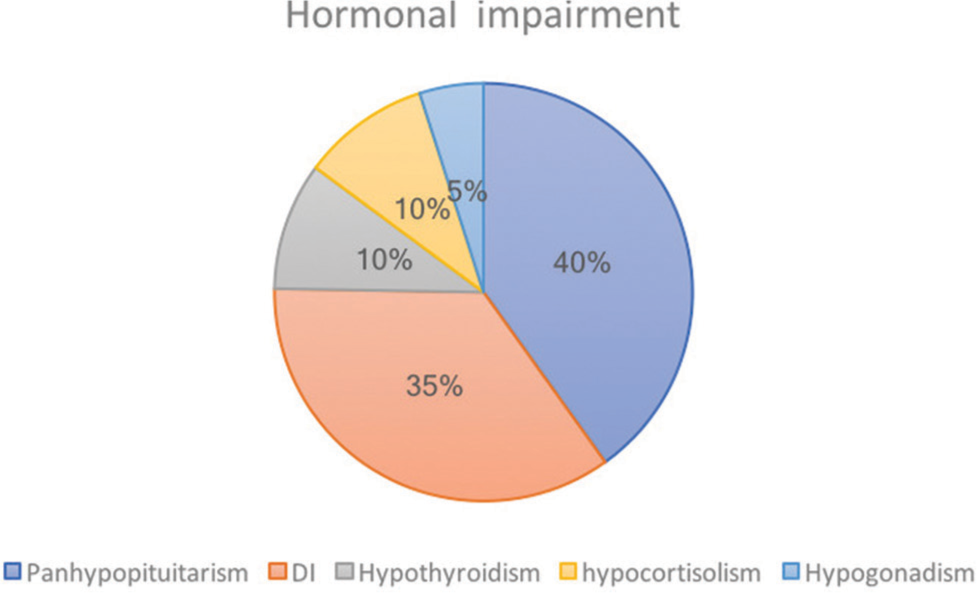
Visual loss and/or field deficits were the most common clinical presentation in 26/30 (86%); headaches were the 2nd most common clinical presentation in 24/30 (80%); 4/30 presented with diplopia (13%). Only 1/30 patient with de novo craniopharyngiomas had hormonal imbalance and 8/30 had visual deficits. Eleven patients had hormonal impairment upon diagnosis of malignant craniopharyngioma (37%). Of these, 8/11 suffered from pan-hypopituitarism (73%), 7/11 from diabetes insipidus (DI) (64%), 2/11 from hypothyroidism (18%), 2/11 from hypocortisolism (18%), and 1/11 from hypogonadism (9%) [
Fifteen patients received radiation therapy before the malignant transformation (50%), and 5/30 received radiation therapy after the diagnosis of malignant craniopharyngioma (17%). There was no statistical significance in radiation therapy before malignant transformation and the rate of benign to malignant transformation (P = 0.379). Patients with a de novo malignant craniopharyngioma were excluded from the analysis to specifically study the benign to malignant transformation with radiation therapy. The median total recurrences were 3 (0–5), and the median total surgeries were 3 (1–5).
Twenty patients underwent a transcranial approach (67%), while 10/30 underwent transsphenoidal surgery (33%). Gross total resection (GTR) was achieved in 2/30 through the transcranial approach. Nine patients received chemotherapy (30%). The most common pathology type was adamantinomatous in 22/30 (73%); the papillary pathology type was found in 2 patients (7%). Six articles did not specify the original pathology type and only commented on the squamous cell histologic appearance. Total patient mortality was 20/30 (67%), with an average time to mortality of 5.3 months ±4.3 (0.1–15). There was no statistical significance between chemotherapy and longer survival (P = 0.115).
DISCUSSION
Malignant craniopharyngioma was first described in 1973 as a de novo squamous carcinoma of the sella turcica.[
The adamantinomatous histology subtype occurs in both adult and pediatric patients and can be morphologically solid or cystic; the papillary subtype almost always develops in adults (34.3%) and is mostly solid in morphology.[
The pathological appearances of a malignant craniopharyngioma are a marked nuclear atypia, a high nucleus-to-cytoplasm ratio, and robust mitotic activity.[
The clinical presentation of benign craniopharyngioma is related to headaches (53%), visual deficits (75%), and diplopia (5%).[
Hormonal deficits of benign craniopharyngioma differ between pediatric and adult patients. Growth hormone deficits are noted in 75% of children and 20% of adults; thyroid-stimulating hormone deficiency is seen in 25% of children and 39% of adults.[
A previous publication showed that radiation therapy of benign craniopharyngiomas does not portend a higher risk of malignant transformation.[
In a systematic review of benign craniopharyngioma surgical resection and outcome, almost 57% of patients had GTR.[
In one study, the overall survival of patients with benign craniopharyngioma was 95% at 2 years, 91% at 5 years, and 83% at 10 years; the progression-free survival was 84% at 2 years, 78% at 5 years, and 60% at 10 years.[
The limitation in this manuscript is related to the very uncommon nature of malignant craniopharyngiomas, and hence, the rare, published case reports. Larger sample size is needed to ideally study the effects of radiation therapy and chemotherapy on malignant transformation and survival. In addition, individual chemotherapeutic agents should be assessed for their efficacy.
CONCLUSION
Malignant craniopharyngioma is a rare entity with few published articles in the literature. It is derived mainly from an adamantinomatous subtype and predominantly occurs in the adult population. There is a robust endocrinologic abnormality manifesting as pan-hypopituitarism, compared to benign craniopharyngiomas. It carries a high mortality rate within a year of diagnosis. Radiation therapy may not slow the rate of benign to malignant transformation, and chemotherapy may not improve survival.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aquilina K, Merchant TE, Rodriguez-Galindo C, Ellison DW, Sanford RA, Boop FA. Malignant transformation of irradiated craniopharyngioma in children: Report of 2 cases. J Neurosurg Pediatr. 2010. 5: 155-61
2. Beer-Furlan A, Abi-Hachem R, Goksel B, Otero JJ, Carrau RL, Prevedello DM. Letter: Radiation-induced malignant transformation of craniopharyngiomas. Neurosurgery. 2016. 79: E313-5
3. Boongird A, Laothamatas J, Larbcharoensub N, Phudhichareonrat S. Malignant craniopharyngioma; case report and review of the literature. Neuropathology. 2009. 29: 591-6
4. Chunhui L, Chuzhong L, Zhenye L, Yilin S, Yazhuo Z. Malignant transformation of radiotherapy-naive craniopharyngioma. World Neurosurg. 2016. 88: 690.e1-5
5. Dandurand C, Sepehry AA, Lari MH, Akagami R, Gooderham P. Adult craniopharyngioma: Case series, systematic review, and meta-analysis. Neurosurgery. 2018. 83: 631-41
6. Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M. Surgical treatment of craniopharyngiomas: Experience with 168 patients. J Neurosurg. 1999. 90: 237-50
7. Fernandez-Miranda JC, Gardner PA, Snyderman CH, Devaney KO, Strojan P, Suarez C. Craniopharyngioma: A pathologic, clinical, and surgical review. Head Neck. 2012. 34: 1036-44
8. Gao S, Shi X, Wang Y, Qian H, Liu C. Malignant transformation of craniopharyngioma: Case report and review of the literature. J Neurooncol. 2011. 103: 719-25
9. Gupta DK, Ojha BK, Sarkar C, Mahapatra AK, Mehta VS. Recurrence in craniopharyngiomas: Analysis of clinical and histological features. J Clin Neurosci. 2006. 13: 438-42
10. Ishida M, Hotta M, Tsukamura A, Taga T, Kato H, Ohta S. Malignant transformation in craniopharyngioma after radiation therapy: A case report and review of the literature. Clin Neuropathol. 2010. 29: 2-8
11. Jaggon J, Abrikian S, Gibson T, Johnson P, Liburd J. Malignant craniopharyngioma: A case report and comprehensive review. Int J Pathol. 2009. 29: 591-6
12. Jeong TS, Yee GT, Kim NR. Malignant transformation of craniopharyngioma without radiation therapy: Case report and review of the literature. J Korean Neurosurg Soc. 2017. 60: 108-13
13. Karavitaki N, Cudlip S, Adams CB, Wass JA. Craniopharyngiomas. Endocr Rev. 2006. 27: 371-97
14. Kristopaitis T, Thomas C, Petruzzelli GJ, Lee JM. Malignant craniopharyngioma. Arch Pathol Lab Med. 2000. 124: 1356-60
15. Lauriola L, Doglietto F, Novello M, Signorelli F, Montano N, Pallini R. De novo malignant craniopharyngioma: Case report and literature review. J Neurooncol. 2011. 103: 381-6
16. Mende KC, Kellner T, Petersenn S, Honegger J, EvangelistaZamora R, Droste M. Clinical situation, therapy, and follow-up of adult craniopharyngioma. J Clin Endocrinol Metab. 2020. 105: dgz043
17. Narla S, Govindraj J, Chandrasekar K, Sushama P. Craniopharyngioma with malignant transformation: Review of literature. Neurol India. 2017. 65: 418-20
18. Negoto T, Sakata K, Aoki T, Orito K, Nakashima S, Hirohata M. Sequential pathological changes during malignant transformation of a craniopharyngioma: A case report and review of the literature. Surg Neurol Int. 2015. 6: 50
19. Nielsen EH, Feldt-Rasmussen U, Poulsgaard L, Kristensen LO, Astrup J, Jorgensen JO. Incidence of craniopharyngioma in Denmark (n=189) and estimated world incidence of craniopharyngioma in children and adults. J Neurooncol. 2011. 104: 755-63
20. Nomura S, Aihara Y, Amano K, Eguchi S, Chiba K, Komori T. A rare case of malignant craniopharyngioma reactive to adjunctive stereotactic radiotherapy and chemotherapy: Case report and literature review. World Neurosurg. 2018. 117: 332-8
21. Plowman PN, Besser GM, Shipley J, Summersgill B, Geddes J, Afshar F. Dramatic response of malignant craniopharyngioma to cis-platin-based chemotherapy. Should craniopharyngioma be considered as a suprasellar “germ cell” tumour?. Br J Neurosurg. 2004. 18: 500-5
22. Rodriguez FJ, Scheithauer BW, Tsunoda S, Kovacs K, Vidal S, Piepgras DG. The spectrum of malignancy in craniopharyngioma. Am J Surg Pathol. 2007. 31: 1020-8
23. Salyer D, Carter D. Squamous carcinoma arising in the pituitary gland. Cancer. 1973. 31: 713-8
24. Signorelli F, D'Alessandris QG, Maira G, Pallini R, Lauretti L. Letter: Malignant craniopharyngioma and radiotherapy: The missing link. Neurosurgery. 2015. 76: E358-9
25. Sklar CA. Craniopharyngioma: Endocrine abnormalities at presentation. Pediatr Neurosurg. 1994. 21: 18-20
26. Sofela AA, Hettige S, Curran O, Bassi S. Malignant transformation in craniopharyngiomas. Neurosurgery. 2014. 75: 306-14
27. Ujifuku K, Matsuo T, Takeshita T, Hayashi Y, Hayashi K, Kitagawa N. Malignant transformation of craniopharyngioma associated with moyamoya syndrome. Neurol Med Chir (Tokyo). 2010. 50: 599-603
28. van Effenterre R, Boch AL. Craniopharyngioma in adults and children: A study of 122 surgical cases. J Neurosurg. 2002. 97: 3-11
29. Virik K, Turner J, Garrick R, Sheehy JP. Malignant transformation of craniopharyngioma. J Clin Neurosci. 1999. 6: 527-30
30. Wang F, He Y, Li C, Wang Y, Zhong L. Malignant craniopharyngioma: A report of seven cases and review of the literature. World Neurosurg. 2020. 135: e194-201
31. Wang W, Chen XD, Bai HM, Liao QL, Dai XJ, Peng DY. Malignant transformation of craniopharyngioma with detailed follow-up. Neuropathology. 2015. 35: 50-5
32. Weiner HL, Wisoff JH, Rosenberg ME, Kupersmith MJ, Cohen H, Zagzag D. Craniopharyngiomas: A clinicopathological analysis of factors predictive of recurrence and functional outcome. Neurosurgery. 1994. 35: 1001-10