- Department of Neurosciences, University Hospital of Wales, Cardiff, United Kingdom.
- Department of Neurosurgery, Southampton General Hospital, Southampton General Hospital, Southampton, United Kingdom.
- Institute of Psychological Medicine and Clinical Neurosciences, School of Medicine, Cardiff University, University Hospital of Wales, United Kingdom.
- Department of Neurosurgery, Cardiff and Vale University Health Board, Cardiff, United Kingdom.
Correspondence Address:
Malik J. Zaben, Department of Neurosurgery, Cardiff and Vale University Health Board, Cardiff, United Kingdom.
DOI:10.25259/SNI_463_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Joseph Merola1, Susruta Manivannan2, Setthasorn Ooi3, Wen Li Chia3, Milan Makwana1, Jozsef Lang1, Paul Leach1, Malik J. Zaben4. The efficacy of cystoperitoneal shunting for the surgical management of intracranial arachnoid cysts in the elderly: A systematic review of the literature. 20-Dec-2021;12:624
How to cite this URL: Joseph Merola1, Susruta Manivannan2, Setthasorn Ooi3, Wen Li Chia3, Milan Makwana1, Jozsef Lang1, Paul Leach1, Malik J. Zaben4. The efficacy of cystoperitoneal shunting for the surgical management of intracranial arachnoid cysts in the elderly: A systematic review of the literature. 20-Dec-2021;12:624. Available from: https://surgicalneurologyint.com/surgicalint-articles/11294/
Abstract
Background: Intracranial arachnoid cysts (AC) are benign, cerebrospinal fluid filled spaces within the arachnoid layer of the meninges. Neurosurgical intervention in children and young adults has been extensively studied, but the optimal strategy in the elderly remains unclear. Therefore, we performed a single center retrospective study combined with a systematic review of the literature to compare cystoperitoneal (CP) shunting with other surgical approaches in the elderly cohort.
Methods: Retrospective neurosurgical database search between January 2005 and December 2018, and systematic review of the literature using PRISMA guidelines were performed. Inclusion criteria: Age 60 years or older, radiological diagnosis of intracranial AC, neurosurgical intervention, and neuroradiological (NOG score)/clinical outcome (COG score). Data from both sources were pooled and statistically analyzed.
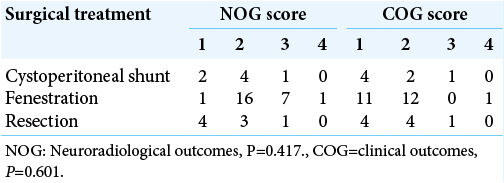
Results: Our literature search yielded 12 studies (34 patients), which were pooled with our institutional data (13 patients). CP shunts (7 patients; 15%), cyst fenestration (28 patients; 60%) and cyst marsupialisation/resection (10 patients; 21%) were the commonest approaches. Average duration of follow-up was 23.6, 26.9, and 9.5 months for each approach, respectively. There was no statistically significant association between choice of surgical intervention and NOG score (P = 0.417), COG score (P = 0.601), or complication rate (P = 0.955). However, CP shunting had the lowest complication rate, with only one patient developing chronic subdural haematoma.
Conclusion: CP shunting is a safe and effective surgical treatment strategy for ACs in the elderly. It has similar clinical and radiological outcomes but superior risk profile when compared with other approaches. We advocate CP shunting as first line neurosurgical intervention for the management of intracranial ACs in the elderly.
Keywords: Cystoperitoneal shunt, Endoscopic fenestration, Intracranial arachnoid cysts, Marsupialisation
INTRODUCTION
Arachnoid cysts are benign, cerebrospinal fluid (CSF)-filled spaces within the arachnoid layer of the meninges. They have a predilection for the middle cranial fossa but can appear in the anterior and posterior cranial fossae and more rarely within the sella or parenchyma.[
Individuals may remain asymptomatic for their entire life despite the apparent expansive behaviour of arachnoid cysts which can, in some instances, cause mass effect on surrounding structures. Symptomatic lesions tend to present with signs and symptoms of raised intracranial pressure, focal neurological deficit, or cortical irritation resulting in seizure activity. The patients may also suffer with neuropsychiatric disturbances, which have been shown to improve following cyst decompression.[
The general consensus among neurosurgeons dictates that surgical decompression is recommended for symptomatic cysts and can lead to improved quality of life.[
MATERIALS AND METHODS
Institutional data
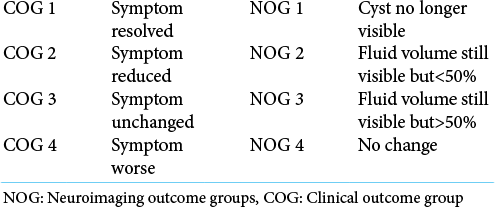
A retrospective review of our local neurosurgical database between January 2005 and December 2018 was performed to identify patients fulfilling the following criteria: (1) age more than or equal to 60 years; (2) radiological diagnosis of arachnoid cyst or histological diagnosis if available; (3) surgical management of arachnoid cyst. The following data were extracted: patient demographics, clinical presentation, anatomical location of cyst, cyst size and volume, Galassi score (if located in the middle cranial fossa), choice of surgical intervention, post-operative complications, clinical/neuroradiological outcomes, and duration of follow-up. Clinical (COG) neuroradiological outcomes (NOG) at follow-up were defined in accordance with the classification system designed by Helland and Wester (2007) [
Table 1:
Helland and Wester (2007)[
Systematic review of the literature
Systematic review of the literature was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[
Search strategy
A multi-database (PubMed, Embase, Web of Science) literature search between January 1980 and May 2020 was performed by authors SO and WC. Conflict of opinion was settled by PL and MZ. Varying combinations of the following search terms were used: arachnoid cyst, cystoperitoneal shunt, endoscopic fenestration, and marsupialization. Only articles in English were included in the study. The bibliographies of identified papers were examined to identify any further relevant articles. All titles and abstracts were reviewed. Eligible studies were included if they satisfied the following criteria: 1) diagnosis of intracranial arachnoid cysts; 2) patient age equal to or >60 years; 3) reported outcomes of surgical intervention including, but not limited to, CP shunt, endoscopic fenestration, and marsupialisation. Any uncertainties were discussed with the authors JM/MZ.
Data extraction/analysis
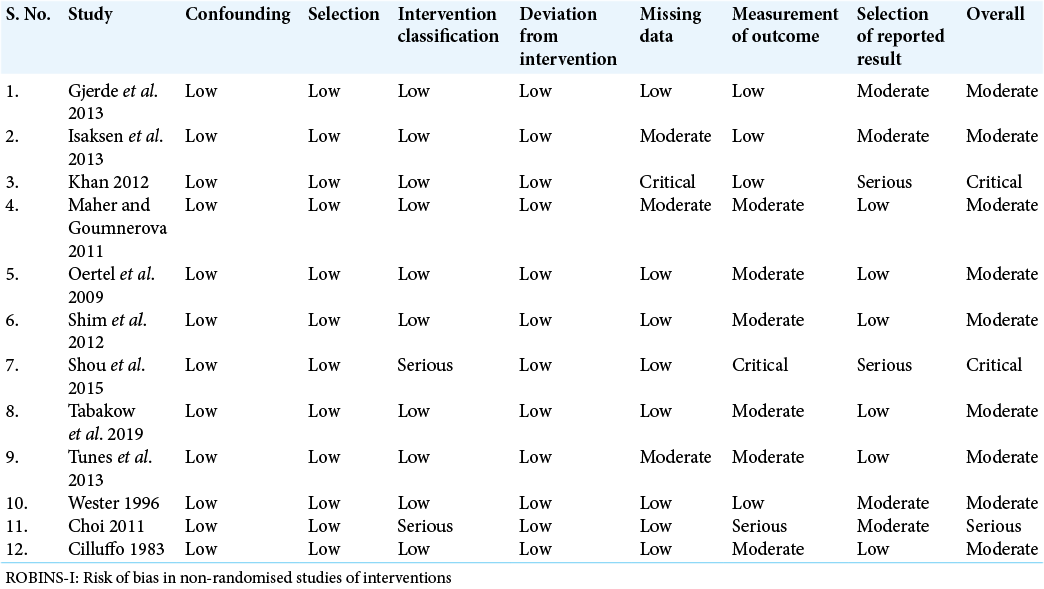
Data were extracted from selected papers and included patient demographics, clinical presentation, anatomical location of cyst, cyst size and volume, Galassi score (if located in the middle cranial fossa), choice of surgical intervention, post-operative complications, clinical/neuroradiological outcomes, and duration of follow-up. Critical appraisal of included studies, and risk of bias was analysed by SM, MM, and JL using an adapted version of the risk of bias in nonrandomised studies of interventions assessment tool.[
Statistical analysis
Baseline characteristics (patient demographics, clinical presentation, cyst location, size, volume) were summarized using descriptive statistics. Continuous variables were reported as means with standard deviation. Outcome data (NOG, COG, complication rates) were analyzed using the Chi-square statistic given the non-parametric distribution of our data. For this purpose, NOG/COG was dichotomized into satisfactory outcomes defined as outcomes 1–2, and unsatisfactory defined as 3–4.
RESULTS
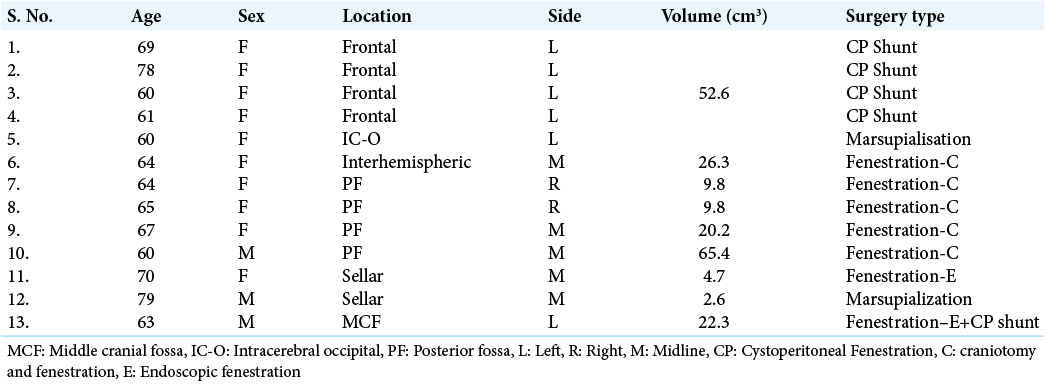
Retrospective analysis of our institutional data yielded a total of 13 patients within the elderly cohort that underwent surgical management of arachnoid cysts. Average age was 66 years, and the majority of patients were female (n = 10; 76.9%). Cyst locations included frontal (n = 4; 30.8%), posterior fossa (n = 4; 30.8%), sellar (n = 2; 15.4%), intracerebral (n = 1; 7.7%), interhemispheric (n = 7.7%), and middle cranial fossa (n = 1; 7.7%). Cysts were either left sided (n = 6; 46.2%), right sided (n = 2; 15.3%), or located in the midline (n = 5; 38.5%). Four patients (30.7%) underwent CP shunt insertion, 6 underwent cyst fenestration (46.2%) – five were through craniotomy and one through endoscope, 2 patients had cyst marsupialisation (15.4%), and 1 patient (7.7%) underwent a mix of endoscopic fenestration and CP shunt insertion [
Following systematic review of the literature, a total of 12 articles were eligible for inclusion, consisting of: 4 observational cohort studies, 4 prospective cohort studies, 3 non-randomised comparative studies, and 1 retrospective study. The majority of include studies were at a moderate risk of bias (n = 9; 75%) [
CP shunting was performed on 7 patients (15%), whilst the majority of patients underwent cyst fenestration (n = 28, 60%). Cyst fenestration was specified as being performed endoscopically (n = 3) or via craniotomy (n = 4) in 7 patients, whilst the remainder were unspecified. Of the remainder, craniotomy and cyst resection or marsupialisation (n = 10; 21%), ventriculoperitoneal shunt insertion (n = 1; 2%), or combined endoscopic fenestration and CP shunting (n = 1; 2%) were performed. The final two patients were excluded from further analysis. The average follow-up duration was 17.8 months (range 0–96 months).
The clinical and radiological outcomes were analyzed according to Helland and Wester (2007) classification[
Cyst resection/marsupialisation had the lowest complication rate at 10% but with the shortest follow-up duration, while fenestration had the highest complication rate at 18% with the longest follow-up duration.
DISCUSSION
It is generally agreed that symptomatic intracranial arachnoid cysts require surgical treatment to alleviate symptoms and prevent progression to permanent neurological deficit. Surgical treatment options include needle aspiration, cyst fenestration, cyst resection/marsupialization, or CP shunting. The optimal strategy remains enigmatic among the neurosurgical community. To date, several studies examine evidence for optimal management in children and young adults, but not in the elderly. Our findings demonstrate that CP shunting is an effective first line management option for management of arachnoid cysts in the elderly.
CP shunting is generally considered safe and effective with relatively low rates of morbidity and mortality. Our findings demonstrate no significant difference in outcome when comparing this approach with fenestration or surgical resection. From studies included in our systematic review, only one patient that underwent CP shunting suffered a complication. This patient developed a chronic subdural hematoma over a 3-year follow-up period and required burr hole drainage. There were, however, no reports of repeat surgery for the arachnoid cyst itself. In contrast, complications of cyst fenestration or resection were more directly related to the procedure. Although development of subdural collections following cyst fenestration is a recognized neurosurgical complication in younger patients, we could not identify any studies reporting this in the elderly. This could be due to underreporting of conservatively managed subdural collections or a true difference in risk of this complication in the elderly. For example, a study by Tunes et al. (2014) reported outcomes from seventeen patients surgically treated for temporal arachnoid cysts, of which four patients were included in our review. Whilst the development of chronic subdural hematoma was reported in three patients, the age of the respective patients was not specified.[
Another concern following treatment of arachnoid cysts is recurrence or failure requiring repeat surgery. Our results demonstrate that repeat surgery was not required in any patients that underwent CP shunting during an average follow-up 23.6 months. However, three patients that required repeat surgery due to recurrence of symptoms had initially undergone cyst fenestration or resection, with an average follow-up 35 months. Further studies are required to elucidate whether this remains true over longer periods of follow-up. A recent review by Hall et al. (2019) reported repeat surgery rates of 24.4% following endoscopic cyst fenestration, and 14.7% after microsurgically treated patients. Only three young patients underwent CP shunting, and one required further surgery due to a chronic subdural hematoma that was also present pre-operatively.[
Although shunt dependence is a post-surgical concern following CP shunting,[
CP shunting of arachnoid cysts has been shown to have similar outcomes in the more widely studied younger population.[
Study limitations
The authors acknowledge limitations of this review. The review of our institutional cohort was retrospective, sample size is small and there is variation in follow-up time. The systematic review included only non-randomised studies and bias assessment indicated moderate to critical bias. Larger randomized trials are needed to address this especially in the context of an ageing population.
CONCLUSION
CP shunting is a safe and effective first line surgical management strategy for symptomatic intracranial arachnoid cysts in the elderly population. Similar clinical and radiological outcomes are demonstrated when compared with cyst fenestration or resection, but a superior risk profile and reduced surgical burden. Considering its less invasive nature, CP shunt may therefore be considered the first line surgical treatment of intracranial ACs.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to acknowledge Health and Care Research Wales for the opportunities provided and for improving health and care of people and communities in Wales.
References
1. Ali ZS, Lang SS, Bakar D, Storm PB, Stein SC. Pediatric intracranial arachnoid cysts: Comparative effectiveness of surgical treatment options. Childs Nerv Syst. 2014. 30: 461-9
2. Benedetti A, Carbonin C, Colombo F. Possible aetiopathogenetic correlation between primary empty sella and arachnoid cyst. Acta Neurochir (Wien). 1977. 38: 269-78
3. Caruso R, Salvati M, Ccrvoni L. Primary intracranial arachnoid cyst in the elderly. Neurosurg Rev. 1994. 17: 195-8
4. Chen Y, Fang HJ, Li ZF, Yu SY, Li CZ, Wu ZB. Treatment of middle cranial fossa arachnoid cysts: A systematic review and meta-analysis. World Neurosurg. 2016. 92: 480-90.e2
5. Chhabra VS, Zhang J, Olson JJ. Association between an arachnoid cyst and intracranial aneurysms misdiagnosed as a cystic tumor with a mural nodule. Neurosurg Focus. 2008. 22: E3
6. Corona-Ruiz JM, de Jesus O. Enlarging temporal arachnoid cyst extending inside the sphenoid sinus. World Neurosurg. 2018. 115: 1-4
7. Couvreur T, Hallaert G, van der Heggen T, Baert E, Dewaele F, Okito JP. Endoscopic treatment of temporal arachnoid cysts in 34 patients. World Neurosurg. 2015. 84: 734-40
8. Dawkins RL, Hackney JR, Riley KO. Penetration of an optic nerve by a sellar/suprasellar arachnoid cyst. World Neurosurg. 2016. 87: 662.e7-11
9. de Oliveira Filho ÍT, Romero PC, Fontoura EA, de Oliveira SD, Botelho RV. Symptomatic foramen of Magendie arachnoid cyst in an elderly patient: The second case report in the literature. Surg Neurol Int. 2019. 10: 189
10. di Gaeta A, Giurazza F, Guarnieri G, Muto M. Giant arachnoid cyst associated with acute subdural haematoma: A case report. Neuroradiol J. 2017. 30: 286-9
11. Feletti A, Alicandri-Ciufelli M, Pavesi G. Transaqueductal trans-magendie fenestration of arachnoid cyst in the posterior fossa. Acta Neurochir (Wien). 2016. 158: 655-62
12. Funaki T, Makino Y, Arakawa Y, Hojo M, Kunieda T, Takagi Y. Arachnoid cyst of the velum interpositum originating from tela choroidea. Surg Neurol Int. 2012. 3: 120
13. Gaberel T, Ponte KF, Khouri S, Emery E. Arachnoid cyst associated to spontaneous CSF fistula and massive pneumocephalus. Acta Neurochir (Wien). 2012. 154: 1941-2
14. Gjerde PB, Schmid M, Hammar Å Wester K. Intracranial arachnoid cysts: Impairment of higher cognitive functions and postoperative improvement. J Neurodev Disord. 2013. 5: 21
15. Graillon T, Metellus P, Adetchessi T, Dufour H, Fuentes S. Adult symptomatic and growing arachnoid cyst successfully treated by ventriculocystostomy: A new insight on adult arachnoid cyst history. Neurochirurgie. 2013. 59: 218-20
16. Güdük M, HamitAytar M, Sav A, Berkman Z. Intrasellar arachnoid cyst: A case report and review of the literature. Int J Surg Case Rep. 2016. 23: 105-8
17. Hall S, Smedley A, Rae S, Mathad N, Waters R, Chakraborty A. Clinical and radiological outcomes following surgical treatment for intra-cranial arachnoid cysts. Clin Neurol Neurosurg. 2019. 177: 42-6
18. Hall S, Smedley A, Sparrow O, Mathad N, Waters R, Chakraborty A. Natural history of intracranial arachnoid cysts. World Neurosurg. 2019. 126: e1315-20
19. Harter LP, Silverberg GD, Brant-Zawadzki M. Intrasellar arachnoid cyst. Neurosurgery. 1980. 7: 387-90
20. Hayashi Y, Kita D, Watanabe T, Yoshikawa A, Hamada JI. Symptomatic foramen of Magendie arachnoid cyst in an elderly patient. Surg Neurol Int. 2015. 6: 7
21. Helland CA, Wester K. A population based study of intracranial arachnoid cysts: Clinical and neuroimaging outcomes following surgical cyst decompression in adults. J Neurol Neurosurg Psychiat. 2007. 78: 1129-35
22. Hendrix P, Senger S, Griessenauer CJ, Simgen A, Linsler S, Oertel J. Preoperative navigated transcranial magnetic stimulation and tractography to guide endoscopic cystoventriculostomy: A technical note and case report. World Neurosurg. 2018. 109: 209-17
23. Hishikawa T, Chikama M, Tsuboi M, Yabuno N. Two cases of symptomatic arachnoid cysts in elderly patients-a comparison and analysis with child cases. No Shinkei Geka. 2002. 30: 959-65
24. Huang D, Abe T, Kojima K, Tanaka N, Watauabe M, Ohkura A. Intracystic hemorrhage of the middle fossa arachnoid cyst and subdural hematoma caused by ruptured middle cerebral artery aneurysm. AJNR Am J Neuroradiol. 1999. 20: 1284-6
25. Huang JH, Mei WZ, Chen Y, Chen JW, Lin ZX. Analysis on clinical characteristics of intracranial arachnoid cysts in 488 pediatric cases. Int J Clin Exp Med. 2015. 8: 18343-50
26. Idris Z, Nandrajog P, Abdullah J, Ghani R, Idris B. Neuronavigation-guided endoscopic and hodotopic approach to an arachnoid cyst. Surg Neurol Int. 2013. 4: 120
27. Isaksen E, Leet TH, Helland CA, Wester K. Maze learning in patients with intracranial arachnoid cysts. Acta Neurochir (Wien). 2013. 155: 841-8
28. Kotil K, Balci N, Bilge T. Intracranial symptomatic giant arachnoid cyst of the interhemispheric fissure presenting with frontal lobe syndrome. Turk Neurosurg. 2007. 17: 147-51
29. Li C, Yin L, Jiang T, Ma Z, Jia G. Shunt dependency syndrome after cystoperitoneal shunting of arachnoid cysts. Childs Nerv Syst. 2014. 30: 471-6
30. Lindvall P, Blomstedt P. Cerebral oedema as a complication following treatment of a giant arachnoid cyst. Acta Neurochir (Wien). 2012. 154: 1417-8
31. Maher CO, Goumnerova L. The effectiveness of ventriculocystocisternostomy for suprasellar arachnoid cysts. J Neurosurg Pediatr. 2011. 7: 64-72
32. Mallucci CL, Jenkinson MD, Conroy EJ, Hartley JC, Brown M, Dalton J. Antibiotic or silver versus standard ventriculoperitoneal shunts (BASICS): A multicentre, single-blinded, randomised trial and economic evaluation. Lancet. 2019. 394: 1530-9
33. Marcoux J, Roy D, Bojanowski MW. Acquired arachnoid cyst after a coil-ruptured aneurysm. Case illustration. J Neurosurgery. 2002. 97: 722
34. Mino M, Fujimura M, Tominaga T. Neuro-endoscopic management of intraparenchymal arachnoid cyst in adults: Three case reports. No Shinkei Geka. 2019. 47: 461-7
35. Miyamoto T, Ebisudani D, Kitamura K, Ohshima T, Horiguchi H, Nagahiro S. Surgical management of symptomatic intrasellar arachnoid cysts-two case reports. Neurol Med Chir (Tokyo). 1999. 39: 941-5
36. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009. 339: b2535
37. Mørkve SH, Helland CA, Amus J, Lund-Johansen M, Wester KG. Surgical decompression of arachnoid cysts leads to improved quality of life: A prospective study. Neurosurgery. 2016. 78: 613-25
38. Oertel JM, Baldauf J, Schroeder HW, Gaab MR. Endoscopic cystoventriculostomy for treatment of paraxial arachnoid cysts. J Neurosurg. 2009. 110: 792-9
39. Ogawa H, Hiroshima S, Kamada K. A case of facial spasm associated with ipsilateral cerebellopontine angle arachnoid cyst. Surg J. 2015. 1: e38-40
40. Okano A, Ogiwara H. The effectiveness of microsurgical fenestration for middle fossa arachnoid cysts in children. Childs Nerv Syst. 2016. 32: 153-8
41. Park KH, Gwak HS, Hong EK, Lee SH. Inflamed symptomatic Sellar arachnoid cyst: Case report. Brain Tumor Res Treat. 2013. 1: 28-31
42. Park KJ, Kang SH, Chae YS, Chung YG. Supratentorial arachnoid cyst located in the brain parenchyma: Case report. Neurosurgery. 2011. 68: E258-62
43. Rabiei K, Jaraj D, Marlow T, Jensen C, Skoog I, Wikkelsø C. Prevalence and symptoms of intracranial arachnoid cysts: A population-based study. J Neurol. 2016. 263: 689-94
44. Ramtahal J. Arachnoid cyst mimicking normal pressure hydrocephalus. A case report and review of the literature. J Neurosurg Sci. 2006. 50: 79-81
45. Riviérez M. Treatment of frontal interhemispheric arachnoid cysts by fenestration of the lamina terminalis. Neurochirurgie. 1993. 39: 311-4
46. Shim KW, Park EK, Lee YH, Kim SH, Kim DS. Transventricular endoscopic fenestration of intrasellar arachnoid cyst. Neurosurgery. 2013. 72: 520-8
47. Shou X, Zhao Y, Li S, Wang Y. Ventriculoscopic surgery for arachnoid cysts in the lateral ventricle: A comparative study of 21 consecutive cases. Int J Clin Exp Med. 2015. 8: 20787-95
48. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016. 355: i4919
49. Sugimoto T, Uranishi R, Yamada T. Gradually progressive symptoms of normal pressure hydrocephalus caused by an arachnoid cyst in the fourth ventricle: A case report. World Neurosurg. 2016. 85: 364.e19-22
50. Suzuki M, Tamaki T, Toda S, Tsuchiya M, Kogure K, Hosone M. Delayed recurrent arachnoid cyst of the occipital convexity in an elderly woman. Neurol Med Chir (Tokyo). 2009. 49: 134-7
51. Tabakow P, Weiser A, Chmielak K, Blauciak P, Bladowska J, Czyz M. Navigated neuroendoscopy combined with intraoperative magnetic resonance cysternography for treatment of arachnoid cysts. Neurosurg Rev. 2020. 43: 1151-61
52. Takai K, Nishihara T, Nemoto S, Ueki K, Miyauchi H, Mishima K. Multilocular cystic lesion associated with a giant aneurysm. J Neurosurg. 2001. 95: 1081
53. Tarantino R, Marruzzo D, Colistra D, Mancarella C, Delfini R. Twelfth nerve paresis induced by an unusual posterior fossa arachnoid cyst: Case report and literature review. Br J Neurosurg. 2014. 28: 528-30
54. Tunes C, Flønes I, Helland C, Wilhelmsen K, Goplen F, Wester KG. Pre-and post-operative dizziness and postural instability in temporal arachnoid cyst patients. Acta Neurol Scand. 2014. 129: 335-42
55. Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP. Incidental findings on brain MRI in the general population. N Engl J Med. 2007. 357: 1821-8
56. Wahl AS, Löffler M, Hausner L, Ruttorf M, Nees F, Frölich L. Case report: A giant arachnoid cyst masking Alzheimer’s disease. BMC Psychiatry. 2019. 19: 274
57. Watanabe M, Kameyama S, Takeda N, Tanaka R. Two cases of symptomatic interhemispheric arachnoid cyst in the elderly. Surg Neurol. 1994. 42: 346-51
58. Weil RJ. Rapidly progressive visual loss caused by a sellar arachnoid cyst: Reversal with transsphenoidal microsurgery. South Med J. 2001. 94: 1118-21
59. Wester K. Peculiarities of intracranial arachnoid cysts: Location, sidedness, and sex distribution in 126 consecutive patients. Neurosurgery. 1999. 45: 775-9
60. Yamasaki F, Kodama Y, Hotta T, Taniguchi E, Eguchi K, Yoshioka H. Interhemispheric arachnoid cyst in the elderly: Case report and review of the literature. Surg Neurol. 2003. 59: 68-74
61. Zhang B, Zhang Y, Ma Z. Long-term results of cystoperitoneal shunt placement for the treatment of arachnoid cysts in children. J Neurosurg Pediatr. 2012. 10: 302-5
62. Zheng J, Wang J, Wang S, Cao Y, Zhao J. Serial observation of an enlarging intracerebral arachnoid cyst. Clin Neurol Neurosurg. 2013. 115: 227-31