- Department of Neurosurgery and Neuroendovascular Therapy, Hospital Universitario Dr. José Eleuterio González, Monterrey, Mexico
- Laboratory of Microsurgical Neuroanatomy, Second Chair of Gross Anatomy, School of Medicine, University of Buenos Aires, San Fernando Hospital, San Fernando, Argentina
Correspondence Address:
Ramon Castruita, Department of Neurosurgery and Neuroendovascular Therapy, Hospital Universitario Dr. José Eleuterio González, Monterrey, Mexico.
DOI:10.25259/SNI_809_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ramon Castruita1, Samuel Perez1, Matias Baldoncini2, Valeria Forlizzi2, Angel Martínez1. The frontal aslant tract: Anatomical description and case report. 01-Nov-2024;15:397
How to cite this URL: Ramon Castruita1, Samuel Perez1, Matias Baldoncini2, Valeria Forlizzi2, Angel Martínez1. The frontal aslant tract: Anatomical description and case report. 01-Nov-2024;15:397. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13195
Abstract
Background: Advances in surgical techniques, neuroimaging, and white matter fiber dissection have facilitated the identification of critical tracts like the frontal aslant tract (FAT) that have garnered attention, despite remaining poorly recognized within the neurosurgical community.
Case Description: We report the case of a 37-year-old male right-handed patient presenting with headache and epilepsy, in whom neuroimaging revealed an intra-axial lesion in the left middle frontal gyrus closely associated with FAT. Successful navigation-guided resection of the lesion was achieved, resulting in a favorable neurological outcome attributable to the preservation of the tract. This case is complemented by a review of the literature and anatomical dissection of FAT in a human specimen.
Conclusion: The FAT has emerged as a critical white matter structure in neurosurgery, given its involvement in speech and motor functions. This case demonstrates the importance of advanced imaging modalities and intraoperative technologies to ensure safe resection.
Keywords: Anatomy, Aslant, Klingler, Oligodendroglioma, Tract, White fibers
INTRODUCTION
Advances in surgical techniques, imaging studies, and white matter fiber dissection have led to the identification of tracts, enabling an understanding of their function and justification for preservation during neurosurgical procedures. One recently notable tract is the frontal aslant tract (FAT), which remains relatively unknown within the neurosurgical community. The FAT is a cerebral white matter tract connecting the superior frontal gyrus (SFG), specifically the presupplementary motor area (pre-SMA) and supplementary motor area (SMA), to the pars opercularis and pars triangularis of the inferior frontal gyrus (IFG) and anterior insula.[
The connectivity between elements involving the FAT, specifically between the pre-SMA and IFG, was only recently discovered in 2007, thanks to advancements in imaging techniques.[
Damage to the FAT during tumor resection can result in relatively subtle neurological deficits.[
CASE DESCRIPTION
A 37-year-old man, right-handed with no significant medical history, presented with a 6-month history of moderate-intensity headache characterized by oppressive symptoms, followed by a bilateral tonic-clonic seizure episode, prompting evaluation. Imaging studies revealed an intra-axial lesion in the left middle frontal gyrus with features suggestive of a low-grade glial tumor [
Figure 1:
Brain magnetic resonance imaging, (a) axial T1-weighted image showing a hypodense lesion in the left middle frontal gyrus with a hyperdense center, accompanied by minimal perilesional edema compressing the left superior and inferior frontal gyri, (b) hyperdense lesion on T2-weighted image, and (c) minimal enhancement with gadolinium contrast on T1-weighted contrast-enhanced sequence.
The patient scored 15 points on the Glasgow coma scale, with pupils at 3 mm, reactive, and no cranial nerve deficits. Motor and sensory functions were preserved with hyperreflexia. Language assessment revealed preserved repetition, naming, and comprehension, as well as phonological skills. Further evaluations with functional magnetic resonance imaging and tractography, focusing on language processing, were performed [
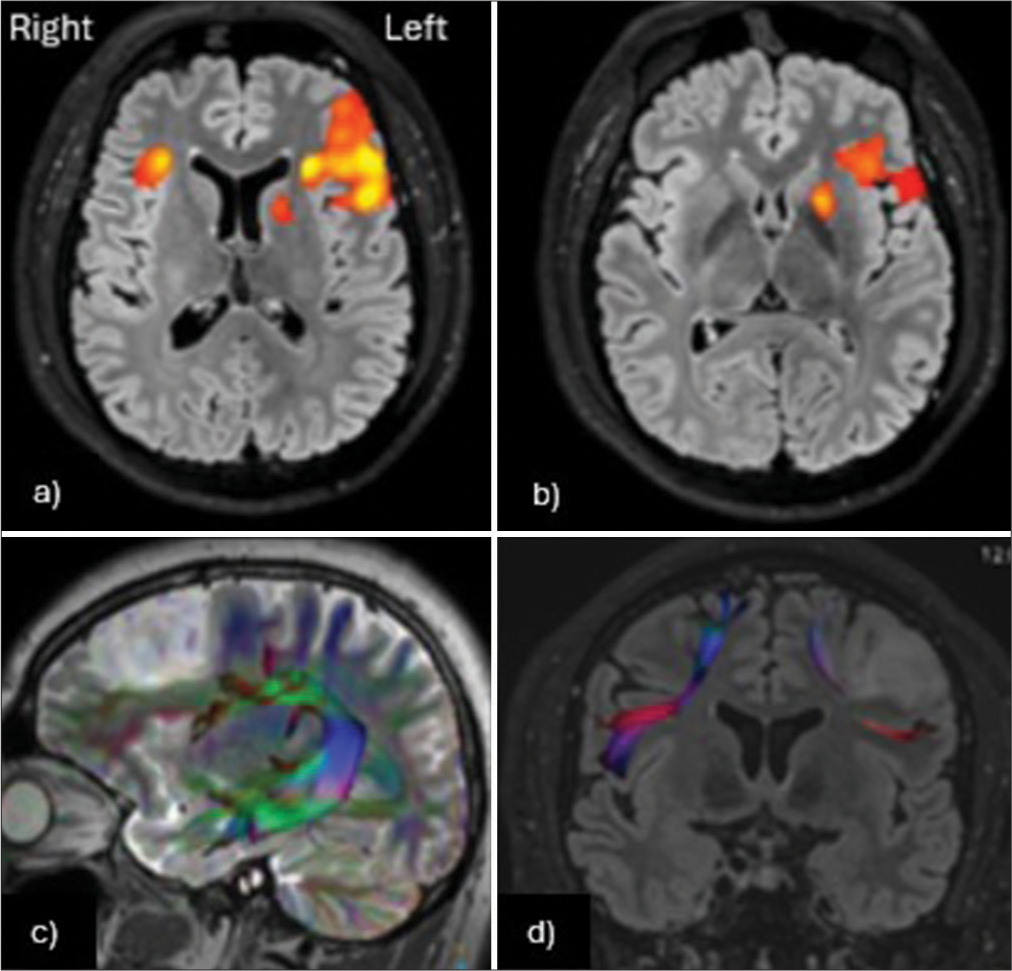
Figure 2:
(a and b) Functional magnetic resonance imaging shows clear left-hemisphere dominance for language functions, with activity in the left inferior frontal gyrus. (c) The left arcuate fasciculus appears intact, with mild caudal displacement due to the lesion. (d) The Aslant tracts are visible, with compression and reduced fiber count on the left side, in close proximity to the lesion.
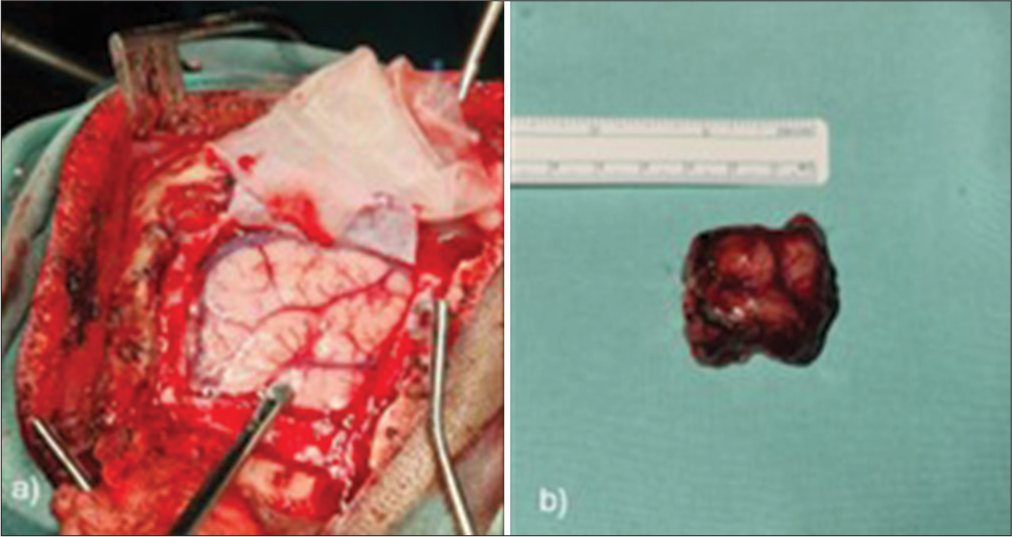
The patient underwent a left frontal craniotomy and tumor resection using neuronavigation, with an intraoperative diagnosis of low-grade glioma consistent with oligodendroglioma. Histopathological examination confirmed grade 2 oligodendroglioma [
RESULTS
The FAT is a white matter fiber tract that courses in the coronal plane, connecting the SFG to the ipsilateral IFG.[
The superior terminations of the FAT are commonly identified in the SMA complex of the SFG, specifically in its medial portion, as well as in the dorsolateral prefrontal cortex of the SFG.[
Using Klingler’s 1935 technique, a post-mortem dissection of white matter fibers was conducted on a right cerebral hemisphere from an adult male autopsy specimen. A right cerebral hemisphere from an adult male was obtained from an autopsy. The brain tissue was fixed in a 10% formalin solution for a few months. Subsequently, the arachnoid and cortical vessels were removed. The cerebral hemisphere was frozen for 10 days. The sample was washed with water, and once thawed, the dissection began with the removal of the cortex and exposure of the U-fibers. The dissection of the white matter was performed as follows: initially, from lateral to medial, starting in the middle frontal gyrus, using wooden spatulas for the initial cortical dissection. Once the U-fibers were identified, metal dissectors with blunt tips were used to complete the dissection. The medial SFG and the pars opercularis/triangularis regions were exposed, revealing the FAT’s oblique fibers and their medial orientation relative to the SFL [
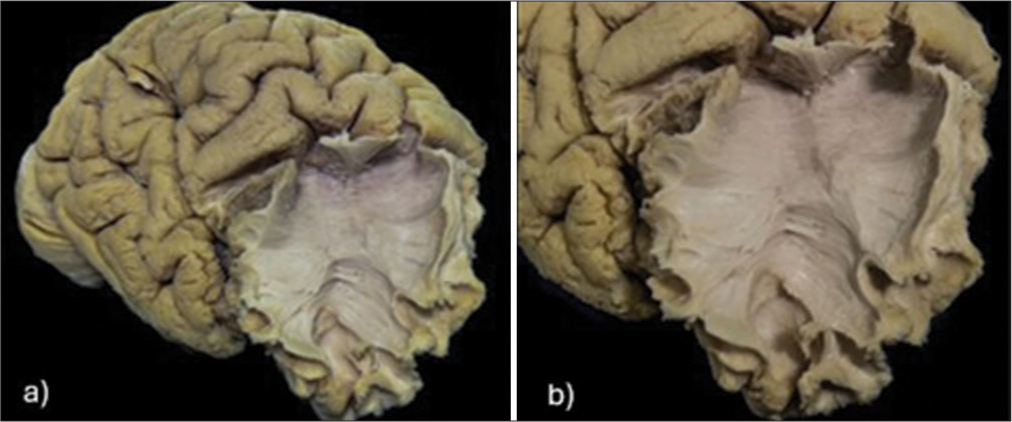
Figure 4:
(a) Dissected human right cerebral hemisphere showing exposure of the frontal aslant tract (FAT) in the frontal lobe. (b) Higher magnification of the same specimen, demonstrating the oblique fiber orientation from the medial superior frontal gyrus to the pars opercularis and pars triangularis, comprising the FAT.
Surgical damage to FAT fibers has been linked to aphasia and speech disturbances, including word-finding difficulties, conversation pauses, and delayed responses, resulting in impaired verbal fluency.[
CONCLUSION
The FAT has emerged as a critical white matter structure in neurosurgery, given its involvement in speech and motor functions. This case demonstrates the importance of advanced imaging modalities and intraoperative technologies to ensure precise and safe resection, avoiding damage to vital tracts like the FAT. Moreover, it emphasizes the value of white matter dissection in specimens for understanding tract anatomy, ultimately enhancing neurosurgical education and training.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted Magnetic Resonance Imaging (MRI) and functional MRI. J Neurosci. 2007. 27: 3743-52
2. Baker CM, Burks JD, Briggs RG, Smitherman AD, Glenn CA, Conner AK. The crossed frontal aslant tract: A possible pathway involved in the recovery of supplementary motor area syndrome. Brain Behav. 2018. 8: e00926
3. Bozkurt B, Yagmurlu K, Middlebrooks EH, Karadag A, Ovalioglu TC, Jagadeesan B. Microsurgical and tractographic anatomy of the supplementary motor area complex in humans. World Neurosurg. 2016. 95: 99-107
4. Catani M, Dell’acqua F, Vergani F, Malik F, Hodge H, Roy P. Short frontal lobe connections of the human brain. Cortex. 2012. 48: 273-91
5. Dick AS, Garic D, Graziano P, Tremblay P. The Frontal Aslant Tract (FAT) and its role in speech, language and executive function. Cortex. 2019. 111: 148-63
6. Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E. A multi-modal parcellation of human cerebral cortex. Nature. 2016. 536: 171-8
7. La Corte E, Eldahaby D, Greco E, Aquino D, Bertolini G, Levi V. The frontal aslant tract: A systematic review for neurosurgical applications. Front Neurol. 2021. 12: 641586
8. Ruan J, Bludau S, Palomero-Gallagher N, Caspers S, Mohlberg H, Eickhoff SB. Cytoarchitecture, probability maps, and functions of the human supplementary and pre-supplementary motor areas. Brain Struct Funct. 2018. 223: 4169-86
9. Szmuda T, Rogowska M, Słoniewski P, Abuhaimed A, Szmuda M, Springer J. Frontal aslant tract projections to the inferior frontal gyrus. Folia Morphol (Warsz). 2017. 76: 574-81
10. Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012. 48: 82-96
11. Young JS, Morshed RA, Mansoori Z, Cha S, Berger MS. Disruption of frontal aslant tract is not associated with long-term postoperative language deficits. World Neurosurg. 2020. 133: 192-5