- Department of Neurosurgery, University Hospital of Geneva, Geneva, Switzerland
- Department of Neurosurgery, Sion Hospital, Sion, Switzerland
- Department of Radiology, Sion Hospital, Sion, Switzerland
- Department of Pathology, Sion Hospital, Sion, Switzerland
- Department of Neuroradiology, University Hospital of Geneva, Geneva University Hospital, Geneva, Switzerland
- Department of Fundamental Neurosciences, Faculty of Medicine, University of Geneva, Geneva, Switzerland.
Correspondence Address:
Daniel Kiss-Bodolay, Department of Neurosurgery, University Hospital of Geneva, Geneva, Switzerland.
DOI:10.25259/SNI_564_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daniel Kiss-Bodolay1, Kyriakos Papadimitriou2, Abderrahmane Hedjoudje3, Christophe Duc4, Maria Isabel Vargas5, Jozsef Zoltan Kiss6, Karl Schaller1, Jean-Yves Fournier2. The interdural hematoma: A subtype of convexity subdural/dural hematoma with specific radioanatomical characteristics. 08-Sep-2023;14:316
How to cite this URL: Daniel Kiss-Bodolay1, Kyriakos Papadimitriou2, Abderrahmane Hedjoudje3, Christophe Duc4, Maria Isabel Vargas5, Jozsef Zoltan Kiss6, Karl Schaller1, Jean-Yves Fournier2. The interdural hematoma: A subtype of convexity subdural/dural hematoma with specific radioanatomical characteristics. 08-Sep-2023;14:316. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12541
Abstract
Background: Rare cases of biconvex hematomas splitting the convexity dura mater were reported and denominated interdural hematoma (IDH). Due to their rarity, little is known about their radiological characteristics, and in most cases, their invasive management with craniotomy and dural membrane excision is unnecessary.
Case Description: We report here a case of single burr-hole endoscopic evacuation of an IDH and its complete resolution after the 6-month follow-up imaging. The literature review reveals 11 reported cases of IDH. Most of them are male and the mean age is 65 years (range 51–90). Most of the reported IDHs were misdiagnosed as epidural hematoma or meningioma, and therefore, they have been managed invasively through craniotomy with dural excision. Diagnosis of the interdural nature was confirmed macroscopically during surgery in all cases and histology was reported for 6 cases. Image analysis found a double dural beak sign and biconvex shape on coronal planes, subarachnoid space enlargement at the collection extremities, and irregular thick inner wall as common radiological aspects of the IDH.
Conclusion: IDH is a rarely reported and often misdiagnosed dural hematoma subtype. Its invasive treatment through craniotomy is likely related to its unknown radiological characteristics. We review and raise awareness about potentially unique radiological anatomy that could avoid unnecessary invasive treatment. Moreover, we report the first case of endoscopically evacuated IDH with long-term follow-up imaging showing complete resolution.
Keywords: Dural beak sign, Dural border cells, Endoscopic evacuation, Interdural hematoma, Radiological anatomy, Subdural hematoma
INTRODUCTION
Chronic subdural hematomas (SDHs) are classically described as crescent shape collections following the hemispheric convexity caused by venous bleeding from bridging veins and are commonly managed through burr-hole evacuation.[
In fact, a minority of all diagnosed SDHs have a biconvex shape with sometimes a very distinct dural beak and 11 cases of such biconvex hematomas were reported in ten publications as being strictly encapsulated between the outer periosteal and the inner thick meningeal layers of the convexity dura mater and named therefore interdural hematoma (IDH) [
Here, we report a case of left convexity IDH that developed following a right subfrontal approach for the resection of a tuberculum sellae meningioma, successfully treated with an endoscopic evacuation. Furthermore, we performed a systematic literature review, with the aim to point out common radiological features that could lead to better identification of this hemorrhagic entity to avoid unneeded aggressive management.
MATERIALS AND METHODS
An electronic literature search in PubMed/MEDLINE, Scopus, Cochrane, and Embase was conducted. The search was done using the term “interdural hematoma.” The literature search strategy was designed to identify all articles in the English language describing cases of adult patients with IDH or cases of biconvex SDH. Moreover, we conducted literature research on the subject of SDH using the term “subdural hematoma” and “subdural hematoma classification(s).” The reference lists in all the identified relevant articles were manually screened for additional relevant references.
RESULTS
Case report
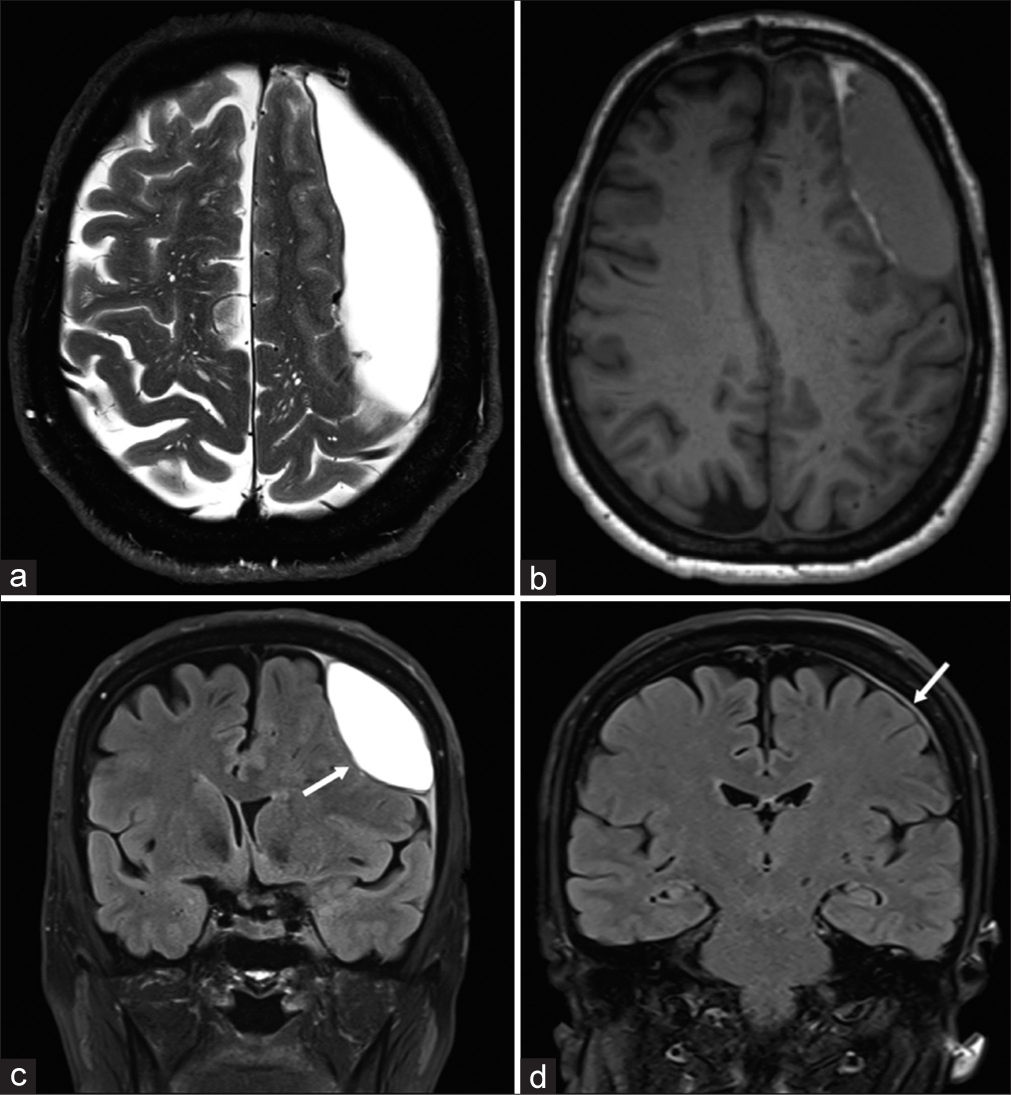
The case is a sexagenarian female operated on for a tuberculum sellae meningioma via a right frontobasal craniotomy with a Simpson grade II resection. The operation was uneventful, and the patient was discharged on postoperative day 5 in stable condition. On the 3-month follow-up, brain magnetic resonance imaging (MRI) revealed a newly formed extra-axial contralateral left convexity hematoma [
Figure 1:
Illustrative case no. 1 of interdural hematoma. (a) Preoperative T2-weighted axial magnetic resonance imaging (MRI); (b) preoperative T1-weighted axial MRI; (c) preoperative T2 fluid attenuated inversion recovery (FLAIR) coronal MRI showing the biconvex lentiform hematoma (white arrow); and (d) 6 months postoperative T2 FLAIR coronal MRI showing the complete resolution of the collection with a slight residual dural thickening (white arrow). Notice the irregular thick inner wall of the hematoma (a). Notice the dural thickening at both dural edges (dural beak sign) indicating dural splitting and the slight subarachnoid space enlargement (c).
Imaging characteristics
On the MRI, the collection was localized over the frontoparietal convexity and was crossing the sutures like a SDH [
Surgical details
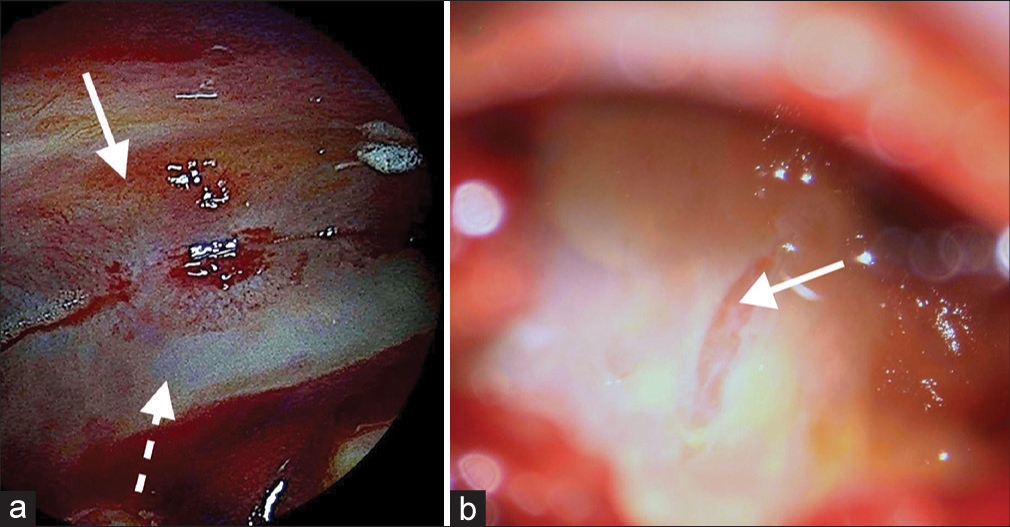
Although the patient was asymptomatic, due to the size of the collection, the midline shift, and its epidural localization, we decided to evacuate the collection. With the aid of neuronavigation (Medtronic Stealth Navigation), a single burr-hole centered on the outer convexity of the hematoma was performed. After drilling the bone, an intact dural membrane was found excluding its epidural origin. After coagulation and incision of the dura, a thin classically looking grayish hematoma membrane was identified. Piercing this membrane resulted in the outflow of a liquid that had a typical chronic blood aspect often described as “motor oil.” After rinsing profusely with saline, the cavity was explored with the help of the neuroendoscope (Karl Storz). An opaque dura-like thick yellowish hemorrhagic membrane was observed under direct vision with irregular darker yellowish spots smoothly in continuity with all of its walls like a proper dural pocket [
Figure 2:
Intracranial, interdural endoscopic pictures taken through a single burr-hole, after hematoma evacuation from case no. 1. (a) Image showing the dural splitting from the inside of the hematoma pocket at the dorsomedial apex (dashed white arrow points to the inner dural layer and solid white arrow to the outer dural layer). (b) Image of the inner wall after dural biopsy, revealing the transparent thin arachnoid membrane (solid white arrow).
Histopathological findings
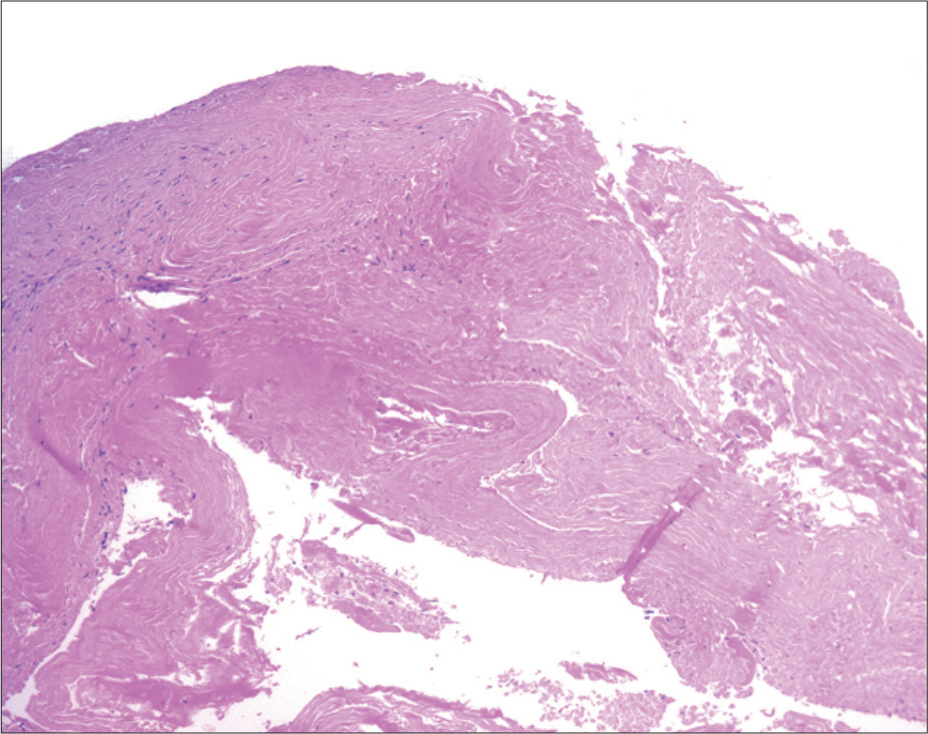
Histopathological examination confirmed the dural origin of the sampled tissue piece coming from the thick inner membrane of the hematoma. Mature fibroblasts and extracellular collagen were visualized [
Follow-up
The 6-month follow-up MRI showed complete resolution of the hematoma with no residual collection [
DISCUSSION
We report here a rare case of successfully treated biconvex hematoma splitting the convexity dura compatible with an IDH. The nomenclature of IDH should be used carefully as this diagnosis relies on specific radiological patterns and the confirmation that the inner wall of the hematoma is of dural origin.[
Physiopathology
The dura mater has long been considered as an avascular, metabolically rather inactive tissue.[
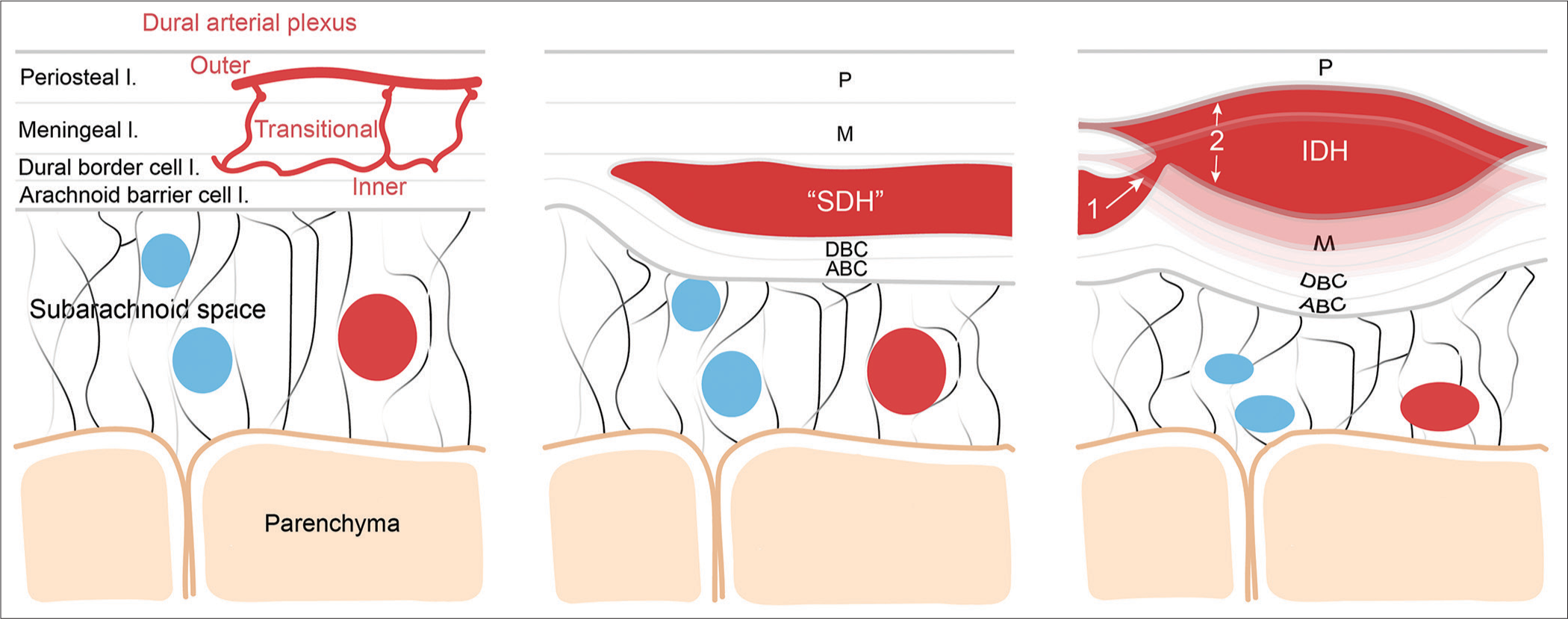
Figure 4:
Schematic representation of the putative pathophysiological mechanisms leading to the formation of the interdural hematoma. Left, schematic illustrating the normal meningeal layer microanatomy including the dural arterial plexus (layer thickness is not scaled). From external to internal meningeal layer: the periosteal layer (P), the meningeal layer (M), the dural border cell layer (DBC), and the arachnoid barrier cell layer (ABC); middle, illustration of the microanatomical localization of the classic subdural hematoma contained by the DBC; Right, the putative mechanism leading to the formation of the IDH through higher pressure blood dissecting more superficially the dural layers from the vascularized inner DBC layer. SDH: Subdural hematoma, IDH: Interdural hematoma.
The precise pathophysiological events leading to the IDH and whether the crescent shape hematoma has any link to the biconvex IDH remains unknown. The accumulation of blood between the border cell and meningeal dural layers from ruptured bridging veins traversing the dura is not the only mechanism responsible for the formation of the crescent shape SDH hematoma.[
It is of interest to note that a meningeal space between the meningeal and periosteal dura, named the interperiosteodural space, part of the extradural neural axis compartment was anatomically demonstrated at the level of the cavernous sinus and its continuity toward the foramen magnum, at the dural folds of the jugular foramen, and the level of the falx cerebri.[
Radioanatomical characteristics
As IDH is a rare and most likely underdiagnosed entity, its radiological features are undefined. Small IDH takes rather the form of the described “Chinese dumpling,” while the more extended frontoparietal convexity IDH as reported by Chen et al. and including our case could be described as uniquely chimeric.[
Attempts were made to subclassify chronic SDH based on its internal radiological anatomy and volumetry at the moment of diagnosis and during its aging with the aim to define predicting factors of its recurrence, among them the Oslo Grading System.[
Surgical management
The optimal surgical management of IDHs is unknown. Likely related to its biconvex shape, more than half of the reported IDH cases, especially the small ones putting the dural layers under tension, were misdiagnosed as an epidural hematoma or a solid tumor and, therefore, managed invasively through a craniotomy [
CONCLUSION
The evident radioanatomical heterogeneity in the shape of the SDH argues against its simple monotype definition. Accumulating observations strongly suggest the existence of a convexity “dural” hematoma spectrum based on its shape, likely related to the amount of dural splitting caused by the hematoma. Early laboratory SDH models pointed out the splitting of the dural border cell layer as a pathophysiological mechanism for the crescent shape SDH.[
Ethics
All procedures performed were in accordance with the 1964 Helsinki Declaration and its later amendments.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using the AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Adeeb N, Mortazavi MM, Tubbs RS, Cohen-Gadol AA. The cranial dura mater: A review of its history, embryology, and anatomy. Childs Nerv Syst. 2012. 28: 827-37
2. Agrawal A. Bilateral biconvex frontal chronic subdural hematoma mimicking extradural hematoma. J Surg Tech Case Rep. 2010. 2: 90-1
3. Alves JL, Santiago JG, Costa G, Pinto AM. A standardized classification for subdural hematomas-I. Am J Forensic Med Pathol. 2016. 37: 174-8
4. Atkinson JL, Lane JI, Aksamit AJ. MRI depiction of chronic intradural (subdural) hematoma in evolution. J Magn Reson Imaging. 2003. 17: 484-6
5. Babayev R, Ekşi MŞ. A blackhole over brain: Interdural hematoma-a challenging diagnosis. Neurol Neurochir Pol. 2015. 49: 189-92
6. Baharvahdat H, Etemadrezaie H, Zabihyan S, Dashti S, Ganjeifar B. Acute interdural hematoma mimicking epidural hematoma: A case report. Turk Neurosurg. 2012. 22: 368-70
7. Bartoli A, Kotowski M, Pereira VM, Schaller K. Acute spinal epidural hematoma and cranial interdural hematoma due to a rupture of a posterior communicating artery aneurysm: Case report. Neurosurgery. 2011. 69: E1000-4 discussion E1004
8. Bechstein M, McDonough R, Fiehler J, Zanolini U, Rai H, Siddiqui A. Radiological evaluation criteria for chronic subdural hematomas: Review of the literature. Clin Neuroradiol. 2022. 32: 923-9
9. Bernard F, Zemmoura I, Cottier JP, Fournier HD, Terrier LM, Velut S. The interperiosteodural concept applied to the jugular foramen and its compartmentalization. J Neurosurg. 2018. 129: 770-8
10. Braun J, Borovich B, Guilburd JN, Zaaroor M, Feinsod M, Grushkiewicz I. Acute subdural hematoma mimicking epidural hematoma on CT. AJNR Am J Neuroradiol. 1987. 8: 171-3
11. Cai Q, Guo Q, Zhang F, Sun D, Zhang W, Ji B. Evacuation of chronic and subacute subdural hematoma via transcranial neuroendoscopic approach. Neuropsychiatr Dis Treat. 2019. 15: 385-90
12. Chang CL, Sim JL, Delgardo MW, Ruan DT, Connolly ES. Predicting chronic subdural hematoma resolution and time to resolution following surgical evacuation. Front Neurol. 2020. 11: 677
13. Chen KT, Huang HC, Lin YJ, Chen MH, Hsieh TC. The relationship between hematoma and pachymeninges in an interdural hematoma: Diagnosis and surgical strategy. World Neurosurg. 2018. 110: 492-8.e3
14. Eom KS, Kim TY, Park JT. Contralateral acute interdural haematoma occurring after burr hole drainage of chronic subdural haematoma. Br J Neurosurg. 2009. 23: 213-5
15. François P, Travers N, Lescanne E, Arbeille B, Jan M, Velut S. The interperiosteo-dural concept applied to the perisellar compartment: A microanatomical and electron microscopic study. J Neurosurg. 2010. 113: 1045-52
16. Friede RL. Incidence and distribution of neomembranes of dura mater. J Neurol Neurosurg Psychiatry. 1971. 34: 439-46
17. Friede RL, Schachenmayr W. The origin ofsubdural neomembranes. II. Fine structural of neomembranes. Am J Pathol. 1978. 92: 69-84
18. Genc A, Yilmaz MB, Egemen E, Yilmaz M, Sav AM. Interdural hematomas: An update of literature. J Neurosurg Sci. 2017. 61: 101-6
19. Haines DE, Harkey HL, Al-Mefty O The. “subdural” space: A new look at an outdated concept. Neurosurgery. 1993. 32: 111-20
20. Haines DE. On the question of a subdural space. Anat Rec. 1991. 230: 3-21
21. Iranmehr A, Namvar M. Traumatic acute convexity interdural hematoma: A case report and literature review. Br J Neurosurg. 2020. 37: 337-9
22. Kawasaki T, Kurosaki Y, Fukuda H, Kinosada M, Ishibashi R, Handa A. Flexible endoscopically assisted evacuation of acute and subacute subdural hematoma through a small craniotomy: Preliminary results. Acta Neurochir (Wien). 2018. 160: 241-8
23. Kim JH, Kang DS, Kim JH, Kong MH, Song KY. Chronic subdural hematoma treated by small or large craniotomy with membranectomy as the initial treatment. J Korean Neurosurg Soc. 2011. 50: 103-8
24. Kolias AG, Chari A, Santarius T, Hutchinson PJ. Chronic subdural haematoma: Modern management and emerging therapies. Nat Rev Neurol. 2014. 10: 570-8
25. Lee KS, Bae WK, Doh JW, Bae HG, Yun IG. Origin of chronic subdural haematoma and relation to traumatic subdural lesions. Brain Inj. 1998. 12: 901-10
26. Lee KS. History of chronic subdural hematoma. Korean J Neurotrauma. 2015. 11: 27-34
27. Liu H, Yan R, Xie F, Richard SA. Hematoma cavity separation and neomembrane thickness are potential triggers of recurrence of chronic subdural hematoma. BMC Surg. 2022. 22: 236
28. Mack J, Squier W, Eastman JT. Anatomy and development of the meninges: Implications for subdural collections and CSF circulation. Pediatr Radiol. 2009. 39: 200-10
29. Maxeiner H, Wolff M. Pure subdural hematomas: A postmortem analysis of their form and bleeding points. Neurosurgery. 2007. 61: 267-72 discussion 272-3
30. Miki S, Fujita K, Katayama W, Sato M, Kamezaki T, Matsumura A. Encapsulated acute subdural hematoma mimicking acute epidural hematoma on computed tomography. Neurol Med Chir (Tokyo). 2012. 52: 826-8
31. Miller JD, Nader R. Acute subdural hematoma from bridging vein rupture: A potential mechanism for growth. J Neurosurg. 2014. 120: 1378-84
32. Miyajima K, Hayashi N, Kurimoto M, Kuwayama N, Hirashima Y, Endo S. Idiopathic interdural hematoma looking like a “Chinese dumpling”--case report. Neurol Med Chir. 2004. 44: 75-6
33. Moshayedi P, Liebeskind DS. Middle meningeal artery embolization in chronic subdural hematoma: Implications of pathophysiology in trial design. Front Neurol. 2020. 11: 923
34. Nabeshima S, Reese TS, Landis DM, Brightman MW. Junctions in the meninges and marginal glia. J Comp Neurol. 1975. 164: 127-69
35. Nagahori T, Nishijima M, Takaku A. Histological study of the outer membrane of chronic subdural hematoma: Possible mechanism for expansion of hematoma cavity. No Shinkei Geka. 1993. 21: 697-701
36. Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001. 95: 256-62
37. Nomura S, Kashiwagi S, Fujisawa H, Ito H, Nakamura K. Characterization of local hyperfibrinolysis in chronic subdural hematomas by SDS-PAGE and immunoblot. J Neurosurg. 1994. 81: 910-13
38. Nouri A, Gondar R, Schaller K, Meling T. Chronic Subdural Hematoma (cSDH): A review of the current state of the art. Brain Spine. 2021. 1: 100300
39. Nunta-Aree S, Paruang T, Sitthinamsuwan B. Timing of brain expansion and recurrence after surgery of chronic subdural hematoma. J Med Assoc Thai. 2017. 100: S59-64
40. Orlin JR, Osen KK, Hovig T. Subdural compartment in pig: A morphologic study with blood and horseradish peroxidase infused subdurally. Anat Rec. 1991. 230: 22-37
41. Park SS, Shin WR, Kim HJ, Kwon CY. Encapsulated unresolved subdural hematoma mimicking acute epidural hematoma: A case report. Korean J Neurotrauma. 2014. 10: 142-5
42. Prieto R, Pascual JM, Subhi-Issa I, Yus M. Acute epidural-like appearance of an encapsulated solid non-organized chronic subdural hematoma. Neurol Med Chir (Tokyo). 2010. 50: 990-4
43. Radcliffe WB, Guinto FC, Adcock DF, Krigman MR. Subdural hematoma shape: A new look at an old concept. Am J Roentgenol. 1972. 115: 72-7
44. Sahyouni R, Goshtasbi K, Mahmoodi A, Tran DK, Chen JW. Chronic subdural hematoma: A historical and clinical perspective. World Neurosurg. 2017. 108: 948-53
45. Sahyouni R, Mahboubi H, Tran P, Roufail JS, Chen JW. Membranectomy in chronic subdural hematoma: Meta-analysis. World Neurosurg. 2017. 104: 418-29
46. Schachenmayr W, Friede RL. The origin of subdural neomembranes. I. Fine structure of the dura-arachnoid interface in man. Am J Pathol. 1978. 92: 53-68
47. Shapiro M, Walker M, Carroll KT, Levitt MR, Raz E, Nossek E. Neuroanatomy of cranial dural vessels: Implications for subdural hematoma embolization. J Neurointerv Surg. 2021. 13: 471-7
48. Stanišic M, Pripp AH. A reliable grading system for prediction of chronic subdural Hematoma recurrence requiring reoperation after initial burr-hole surgery. Neurosurgery. 2017. 81: 752-60
49. Tanaka Y, Ohno K. Chronic subdural hematoma-an up-to-date concept. J Med Dent Sci. 2013. 60: 55-61
50. Talbert DG. The subdural myth: Space or place?. Anat Physiol. 2016. 6: 1000242
51. Tsutsumi S, Ono H, Ishii H, Yasumoto Y. Interdural high signal on CISS sequence: An alternative CSF pathway?. Childs Nerv Syst. 2019. 35: 487-91
52. Unterhofer C, Freyschlag CF, Thomé C, Ortler M. Opening the internal hematoma membrane does not alter the recurrence rate of chronic subdural hematomas: A prospective randomized trial. World Neurosurg. 2016. 92: 31-6
53. Yamashima T, Yamamoto S. Clinicopathological study of acute subdural haematoma in the chronic healing stage. Clinical, histological and ultrastructural comparisons with chronic subdural haematoma. Neurochirurgia (Stuttg). 1984. 27: 98-105
54. Yoo M, Kim JS, Jin SC, Lee SI. Idiopathic interdural hematoma in adult: A case report. NMC Case Rep J. 2016. 3: 103-5