- Department of Neurology and Neurosurgery, Federal University of São Paulo, São Paulo, Brazil.
Correspondence Address:
Feres Chaddad-Neto, Department of Neurology and Neurosurgery, Federal University of São Paulo, São Paulo, Brazil.
DOI:10.25259/SNI_37_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Talita Helena Martins Sarti, Marcos Devanir Silva da Costa, Daniel Paz Araujo, Rodrigo Akira Watanabe, Samuel Tau Zymberg, Ítalo Capraro Suriano, Sergio Cavalheiro, Feres Chaddad-Neto. The long-term effect on functional outcome of endoscopic brainwashing for intraventricular hemorrhage compared to external ventricular drainage alone: A retrospective single-center cohort study. 29-Mar-2024;15:109
How to cite this URL: Talita Helena Martins Sarti, Marcos Devanir Silva da Costa, Daniel Paz Araujo, Rodrigo Akira Watanabe, Samuel Tau Zymberg, Ítalo Capraro Suriano, Sergio Cavalheiro, Feres Chaddad-Neto. The long-term effect on functional outcome of endoscopic brainwashing for intraventricular hemorrhage compared to external ventricular drainage alone: A retrospective single-center cohort study. 29-Mar-2024;15:109. Available from: https://surgicalneurologyint.com/surgicalint-articles/12830/
Abstract
Background: Intraventricular hemorrhage (IVH) is a complex condition with both mechanical and chemical effects, resulting in mortality rates of 50–80%. Recent reports advocate for neuroendoscopic treatment, particularly endoscopic brainwashing (EBW), but long-term functional outcomes remain insufficiently explored. This study aims to outline the step-by-step procedure of EBW as applied in our institution, providing results and comparing them with those of external ventricular drainage (EVD) alone.
Methods: We performed a retrospective analysis of adult patients with IVH who underwent EBW and patients submitted to EVD alone at our institution. All medical records were reviewed to describe clinical and radiological characteristics.
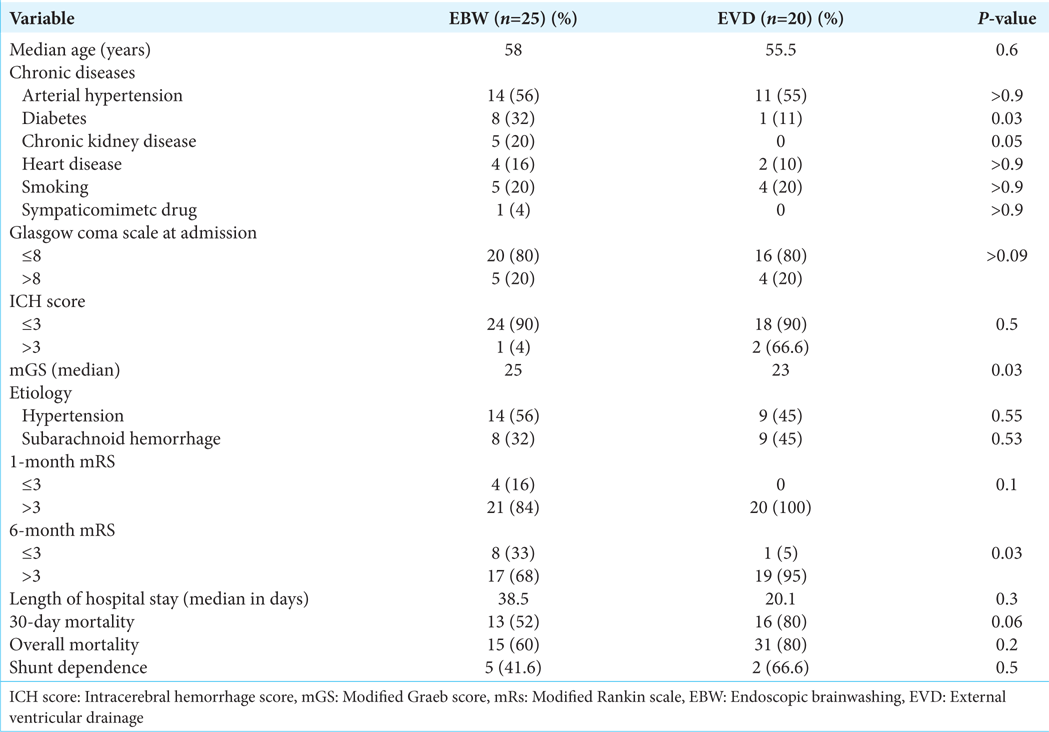
Results: Although both groups had similar baseline factors, EBW patients exhibited a larger hemoventricle (median Graeb score 25 vs. 23 in EVD, P = 0.03) and a higher prevalence of chronic kidney disease and diabetes. Short-term mortality was lower in EBW (52% and 60% at 1 and 6 months) compared to EVD (80% for both), though not statistically significant (P = 0.06). At one month, 16% of EBW patients achieved a good outcome (Modified Rankin scale
Conclusion: Comparing EBW and EVD, patients submitted to the former treatment have the highest modified Graeb scores and, at a long-term follow-up, have better outcomes, demonstrated by the improvement of the patients in the follow-up.
Keywords: Brainwashing, Hypertension, Intraventricular hemorrhage, Neuroendoscopy, Stroke
INTRODUCTION
Intraventricular hemorrhage (IVH) is a multifactorial condition. It may be traumatic or, more commonly, secondary to systemic arterial hypertension, arteriovenous malformation or aneurysm rupture, coagulopathy, vasculitis, tumor hemorrhage, or Moyamoya disease.[
IVH causes an acute increase in intracranial pressure, which leads to secondary brain injury. The harmful effect of IVH is not purely mechanical, as it has a neurotoxic reaction when triggering the inflammatory cascade.[
The surgical treatment of IVH consists of ventricular drainage exclusively, ventricular drainage with fibrinolysis, and neuroendoscopy. The sole insertion of ventricular drainage is technically easy, does not require many instruments and equipment, is widely available, and allows intracranial pressure monitoring. However, it does not act on the neurotoxic effect of the clot and may obstruct and increase the risk of infection. Thus, the effects of attempting to remove IVH with alteplase administered through the external ventricular drain were studied. It is a safe method for specific conditions, such as low-volume supratentorial hemorrhage, stable clot, no severe disability, and no associated cause (aneurysm, arteriovenous malformation, and coagulopathy). However, this treatment did not substantially improve functional outcomes.[
In 1985, endoscopic evacuation of IVH was described.[
There is a lack of information related to the long-term functional outcomes of intraventricular endoscopic brainwashing (EBW) treatment. Thus, we intend to describe our approach to this condition as well as the surgical procedure step by step, serving as a guide to similar centers. We also reported our results with the method and compared it with the sole implantation of an external ventricular drain in patients who presented IVH. In view of the above, we aim to describe our approach in patients presenting with IVH and to provide a step-by-step guide for neuro EBW for this condition. We also reported our clinical and radiological results with the procedure and compared them with solely EVD.
MATERIALS AND METHODS
We performed a retrospective analysis of adult (age >18 years) patients who underwent EBW for IVH and patients who underwent EVD alone at the Department of Neurosurgery of the University of São Paulo Hospital. At least one member of the surgical team was involved in all neuro EBW procedures.
All medical records were reviewed to determine patient demography and outcome: sex, age, comorbidities, clinical presentation; level of consciousness graded by the Glasgow Coma Scale (GCS) before and after surgery; the timing of operation; disability graded by the modified Rankin scale (mRS) 1 week, one month and six months after surgery; and ventricular shunt dependence.
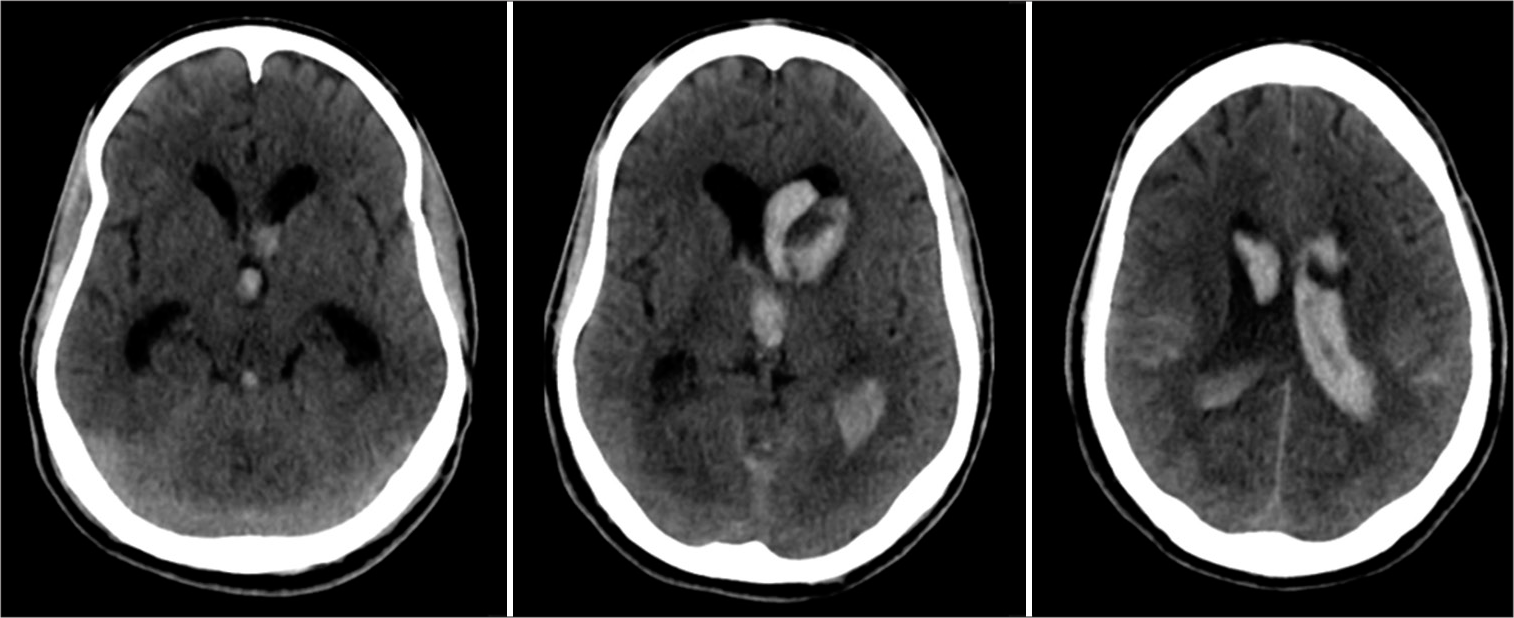
Radiological findings were analyzed through computed tomography (CT). The severity of ventricular hemorrhage was graded according to the modified Graeb score.[
The exclusion criteria were patients with any data missing from the medical records, patients who underwent craniotomy or conservative treatment, and patients presenting with IVH resulting from cerebellar and brainstem hemorrhage.
Qualitative variables were analyzed by contingency tests using Fisher‘s exact test, depending on the sample size and the adequacy of the normal distribution for each set of samples. The Mann–Whitney test was used to analyze quantitative variables if the samples had a distribution pattern that was not normal. An α ≤ 0.05 was considered significant. Data analysis and graph creation were performed using GraphPad Prism 8 for macOS version 8.0.1.
Operation and technical nuances
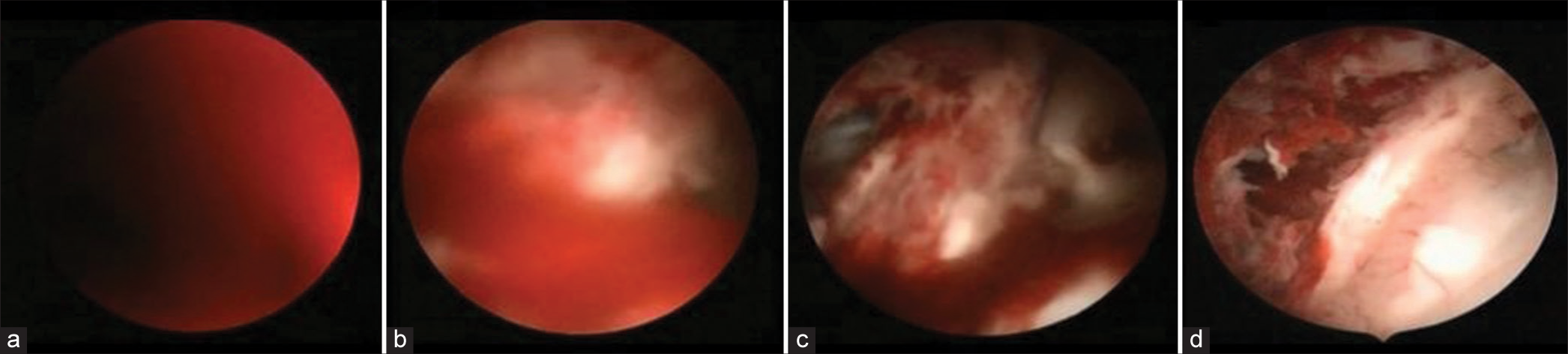
The same neurosurgical team performed all EBW procedures in a standardized manner. We started by evaluating the following aspects of the CT scan [
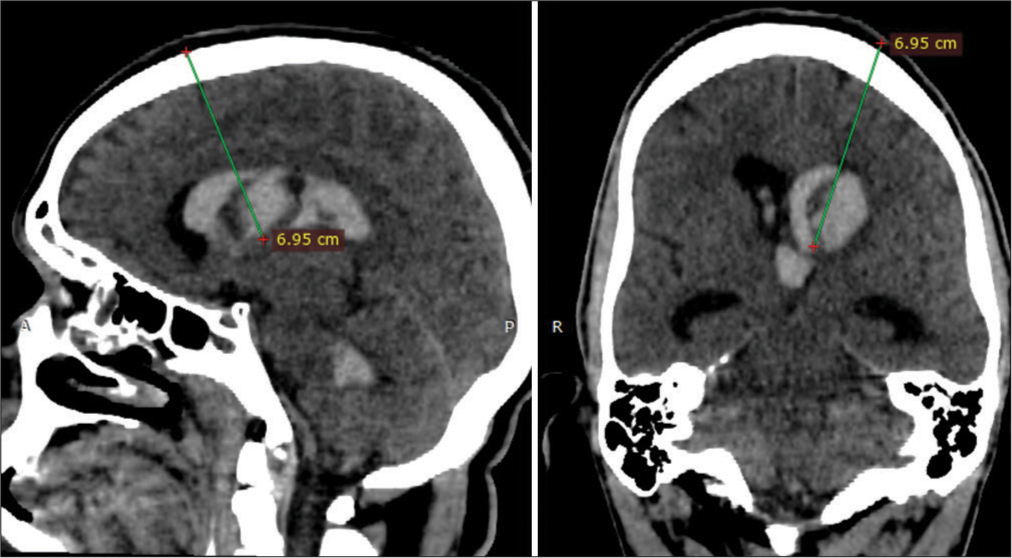
The amount of blood in each of the lateral ventricles: It guides the choice of the laterality of the approach If the blood extends to the third ventricle, It is important to enter the third ventricle to remove as much of the clot as possible and to perform a third ventriculostomy. Identification of the coronal suture: It is of most importance to identify the coronal suture and to calculate its distance from the nasion to proceed to trepanation at the Kocher point Calculate the trajectory: The intraventricular clot distorts the ventricular anatomy and prevents the identification of the structures. Thus, an accurate preoperative calculation allows for a secure endoscope introduction [
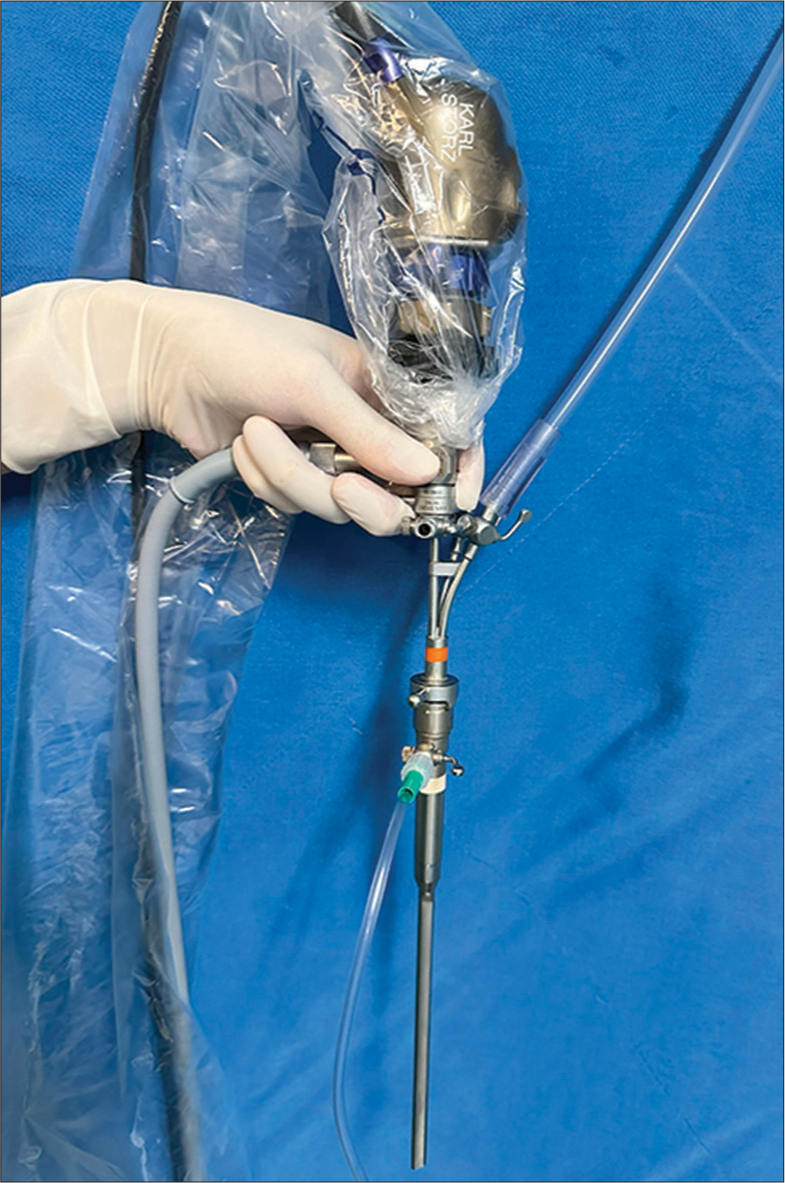
We performed neuroendoscopy with a rigid endoscope (Decq or Gaab lens 30°). The necessary equipment also includes Fogarty catheters 4F and 5F, approximately 12 L of warm lactate ringer or saline solution 0.9%, and two suction hoses connected separately. The necessary equipment is set up as demonstrated in
Figure 3:
The endoscope is disposable, as demonstrated here. The distal overture received continuous saline or lactate irrigation. A hose is connected to the proximal overture at the working channel, and intermittent aspiration is made during large clot cleaning. If both ventricles were extensively inundated, we repeated the procedure on the other side. If not, we performed a septostomy to communicate with both lateral ventricles. At
Each patient was positioned prone with the head flexed enough to place the endoscope entry point at the highest [
A rigid endoscope was used for all procedures, and the cerebral aqueduct and the fourth ventricle could not be accessible as with flexible endoscopes.[
We cannulated the ventricle with an introducer and sheath approximately 3–5 cm in depth (this value varied according to the previously calculated trajectory) until the corpus callosum was pierced. After entraining the lateral ventricle, the endoscope optic was coupled to the sheath. At this point, there was no anatomical landmark due to the clots in the ventricle [
At this point, the foramen of Monro should be well identified. We advanced the endoscope into the third ventricle, and the steps performed at the lateral ventricle were repeated. The clots at this ventricle were not as adherent to the walls as in the lateral ventricle, and they cleared faster. It is necessary to evaluate two main points of obstruction: the foramen of Monro and the entrance to the cerebral aqueduct. If needed, aspiration with a Fogarty 5F catheter was attempted at these tender and dangerous regions. When the floor of the third ventricle was adequately visible, we proceeded with the third ventriculostomy by perforating the tuber cinereum and ballooning with a Fogarty catheter number 4 Fr.
If both ventricles were extensively inundated, we repeated the procedure on the other side. If not, we performed a septostomy to communicate with both lateral ventricles. At the end of the surgery, we introduced a ventricular catheter working as an external ventricular drain and intracranial pressure monitoring. We set the drain up to 10 mmHg.
RESULTS
Forty-seven patients with IVH treated surgically in our department between May 2013 and March 2023 were included in our study. The patients’ mean age was 55 years (range 18–79). Thirty-six patients (80%) presented with GCS < 9.
IVH was due to hypertension in 23 patients (51.1%), spontaneous subarachnoid hemorrhage secondary to aneurysm rupture in 17 patients (37.7%), and other etiologies, such as vasculitis, sinus venous thrombosis, and sympathomimetic drug abuse, in 5 patients (11.1%). Concerning the anatomical location of hemorrhage, the thalamus was most common based on the CHARTS instrument. The median mGS was 25.
Twenty-five patients were treated with EBW and 20 with EVD. There were no differences between the EBW and EVD groups regarding age, arterial hypertension, heart disease, dyslipidemia, sympathomimetic drug abuse, smoking, and GCS score. The EBW patients were more likely to have chronic kidney disease (20% EBW group vs. 0 EVD group, P = 0.05) and diabetes (32% EBW group vs. 5% EVD group, P = 0.03). The demographic, clinical, and radiological characteristics of the patients from each group are provided in
When comparing mGS from the EBW and EVD groups, the differences were statistically significant (P = 0.03). The patients who had EBW exhibited bulkier hemoventricle, with a median mGS of 25. The EVD group had a median mGS of 23.
Regarding the patients who had EBW, the mortality at 1 and 6 months was 52% and 60%, respectively, while that of the patients who underwent EVD surgery was 80% and 80%, with no significant difference between the mortality curves. There was a tendency (not statistically significant, P = 0.06) to have a lower short-term mortality rate in the EBW group. The average hospital stay after the operation was 38.5 days in the EBW group and 20.1 days in the EVD group, with no significant difference between them. A good outcome was considered when mRS ≤3, and in a short-term follow-up (1 month), it was achieved by 4 (16%) patients from the EBW group and 0 from the EVD group (P = 0.1). In the long-term follow-up, a good outcome (mRS ≤ 3) was achieved by 8 (32%) patients from the EBW group and 1 (11%) patient from the EVD group (P = 0.03).
Comparing the patients who had EBW and the patients who had EVD, there was no significant difference in shunt dependency between the groups. Forty-two per cent of the patients who underwent EBW became shunt dependent, and 66% who underwent EVD became shunt dependent as well.
DISCUSSION
Isolated spontaneous IVH is a rare condition. IVH is usually associated with subarachnoid hemorrhage or intracerebral hematoma, presenting worse outcomes and an increased mortality rate when secondary to those conditions. The mortality rate has been reported to be 72% when combined with supratentorial ICH and 50–80% when isolated.[
Intraventricular hematoma has direct mechanical effects by impeding cerebrospinal fluid circulation and is also a chemical process that culminates with an inflammatory response and neurotoxin release.[
EVD is a simple and fast procedure that promptly normalizes intraventricular pressure; however, it does not affect the neurotoxic consequences of IVH. EVD combined with a fibrinolytic agent has already been proposed. Although it has been shown to be safe, it may be associated with infection and rebleeding. Moreover, it did not satisfactorily (at least 80%) remove the intraventricular clot or improve functional outcome (modified Rankin ≤3 scale).[
Neuroendoscopic procedures for the treatment of IVH are not a novelty.[
The EBW group had a modified Graeb score significantly higher than the EVD group (median value of 25 vs. 23, P = 0.03). This indicates that patients who underwent brainwashing surgery had worse IVH than patients who underwent EVD. It isn’t easy to compare this result with the previous studies reported because they calculated the intraventricular inundation by the Graeb score, and we reported our results based on the modified Graeb score. We encourage new studies to use the mGS, which is more closely related to IVH volume and outcome than the Graeb score.[
Studies that reported the outcome after neuroendoscopic surgery showed a favorable outcome, measured by the Glasgow Outcome Scale (GOS).[
When analyzing shunt dependency, our study did not find significant differences between the groups. This is the opposite of our initial hypothesis because the washout technique allows mechanical removal of the clot, and other studies have demonstrated a significant reduction in permanent shunt. The shunt dependency rates were reduced more than 4–5 times in some studies.[
Our study has some limitations. It was a retrospective and single-center study. Many patients could not be included due to the lack of data in the medical records. In addition, the data collection of 45 patients and a heterogeneous sample has significant limitations. However, it was the first national experience reported in the literature to the best of our knowledge.
CONCLUSION
The present study described our surgical planning and technical nuances. Besides some limitations, we demonstrated that patients with IVH are severely clinically compromised at admission (GCS < 9). Comparing EBW and EVD, patients who had the former treatment have the highest modified Graeb scores and, at a long-term follow-up, have better outcomes, as demonstrated by the improvement of the patients in the follow-up.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The authors would like to thank Ph.D. Raphael Wuo-Silva for his contribution to the editing and final review of this manuscript.
References
1. Adams RE, Diringer MN. Response to external ventricular drainage in spontaneous intracerebral hemorrhage with hydrocephalus. Neurology. 1998. 50: 519-23
2. Auer LM. Endoscopic evacuation of intracerebral haemorrhage: High-tecsurgical treatment-a new approach to the problem?. Acta Neurochir (Wien). 1985. 74: 124-8
3. Backlund EO, von Holst H. Controlled subtotal evacuation of intracerebral haematomas by stereotactic technique. Surg Neurol. 1978. 9: 99-101
4. Basaldella L, Marton E, Fiorindi A, Scarpa B, Badreddine H, Longatti P. External ventricular drainage alone versus endoscopic surgery for severe intraventricular hemorrhage: A comparative retrospective analysis on outcome and shunt dependency. Neurosurg Focus. 2012. 32: E4
5. Brott T, Thalinger K, Hertzberg V. Hypertension as a risk factor for spontaneous intracerebral hemorrhage. Stroke. 1986. 17: 1078-83
6. Charidimou A, Schmitt A, Wilson D, Yakushiji Y, Gregoire SM, Fox Z. The cerebral haemorrhage anatomical rating instrument (CHARTS): Development and assessment of reliability. J Neurol Sci. 2017. 372: 178-83
7. Chen CC, Liu CL, Tung YN, Lee HC, Chuang HC, Lin SZ. Endoscopic surgery for intraventricular hemorrhage (IVH) caused by thalamic hemorrhage: Comparisons of endoscopic surgery and external ventricular drainage (EVD) surgery. World Neurosurg. 2011. 75: 264-8
8. Costa MD, Lopes RR, Serrato-Avila JL, Cavalheiro S, ChaddadNeto F. Endoscopic brainwash after clipping a ruptured aneurysm of the communicating segment of the intracranial carotid artery. Surg Neurol Int. 2020. 11: 396
9. Daverat P, Castel JP, Dartigues JF, Orgogozo JM. Death and functional outcome after spontaneous intracerebral hemorrhage. A prospective study of 166 cases using multivariate analysis. Stroke. 1991. 22: 1-6
10. Fiorindi A, Saraceno G, Zanin L, di Bergamo LT, Feletti A, Doglietto F. Endoscopic evacuation of massive intraventricular hemorrhages reduces shunt dependency: A meta-analysis. Asian J Neurosurg. 2022. 17: 541-6
11. Gaberel T, Magheru C, Emery E. Management of non-traumatic intraventricular hemorrhage. Neurosurg Rev. 2012. 35: 485-94
12. Goh KY, Hsiang JN, Zhu XL, Poon WS. Intraventricular recombinant tissue plasminogen activator for treatment of spontaneous intraventricular haemorrhage in pregnancy. J Clin Neurosci. 1999. 6: 158-9
13. Hamada H, Hayashi N, Kurimoto M, Umemura K, Nagai S, Kurosaki K. Neuroendoscopic removal of intraventricular hemorrhage combined with hydrocephalus. Minim Invasive Neurosurg. 2008. 51: 345-9
14. Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR. Thrombolytic removal of intraventricular hemorrhage in treatment of severe stroke: Results of the randomized, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. 2017. 389: 603-11
15. Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001. 32: 891-7
16. Horvath Z, Veto F, Balas I, Kover F, Doczi T. Biportal endoscopic removal of a primary intraventricular hematoma: Case report. Minim Invasive Neurosurg. 2000. 43: 4-8
17. Johnson JR, Idris Z, Abdullah JM, Alias A, Haspani MS. Prevalence of shunt dependency and clinical outcome in patients with massive intraventricular haemorrhage treated with endoscopic washout and external ventricular drainage. Malays J Med Sci. 2017. 24: 40-6
18. Kamikawa S, Inui A, Kobayashi N, Tamaki N, Yamadori T. Intraventricular hemorrhage in neonates: Endoscopic findings and treatment by the use of our newly developed Yamadori-type 8 ventriculoscope. Minim Invasive Neurosurg. 2001. 44: 74-8
19. Li N, Liu YF, Ma L, Worthmann H, Wang YL, Wang YJ. Association of molecular markers with perihematomal edema and clinical outcome in intracerebral hemorrhage. Stroke. 2013. 44: 658-63
20. Li Y, Zhang H, Wang X, She L, Yan Z, Zhang N. Neuroendoscopic surgery versus external ventricular drainage alone or with intraventricular fibrinolysis for intraventricular hemorrhage secondary to spontaneous supratentorial hemorrhage: A systematic review and meta-analysis. PLoS One. 2013. 8: e80599
21. Longatti PL, Fiorindi A, Martinuzzi A. Neuroendoscopic aspiration of hematocephalus totalis: Technical note. Neurosurgery. 2005. 57: E409
22. Longatti PL, Martinuzzi A, Fiorindi A, Maistrello L, Carteri A. Neuroendoscopic management of intraventricular hemorrhage. Stroke. 2004. 35: e35-8
23. Mayfrank L, Kissler J, Raoofi R, Delsing P, Weis J, K€uker W. Ventricular dilatation in experimental intraventricular hemorrhage in pigs. Characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke. 1997. 28: 141-8
24. Morgan T, Awad I, Keyl P, Lane K, Hanley D. Preliminary report of the clot lysis evaluating accelerated resolution of intraventricular hemorrhage (CLEAR-IVH) clinical trial. Acta Neurochir Suppl. 2008. 105: 2017-20
25. Morgan TC, Dawson J, Spengler D, Lees KR, Aldrich C, Mishra NK. The Modified Graeb Score: An enhanced tool for intraventricular hemorrhage measurement and prediction of functional outcome. Stroke. 2013. 44: 635-41
26. Mould WA, Carhuapoma JR, Muschelli J, Lane K, Morgan TC, McBee NA. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke. 2013. 44: 627-34
27. Nakano T, Ohkuma H, Ebina K, Suzuki S. Neuroendoscopic surgery for intracerebral haemorrhage: Comparison with traditional therapies. Minim Invasive Neurosurg. 2003. 46: 278-83
28. Neki H, Shibata A, Komine H, Kohyama S, Yamane F, Ishihara S. Use of flexible endoscopic aspiration for an intraventricular small floating clot with hemorrhage: A technical note. Neurosurg Rev. 2021. 44: 2363-7
29. Oertel JM, Mondorf Y, Baldauf J, Schroeder HW, Gaab MR. Endoscopic third ventriculostomy for obstructive hydrocephalus due to intracranial hemorrhage with intraventricular extension. J Neurosurg. 2009. 111: 1119-26
30. Oka K, Go Y, Yamamoto M, Kumate S, Tomonaga M. Experience with an ultrasonic aspirator in neuroendoscopy. Minim Invasive Neurosurg. 1999. 42: 32-4
31. Rainov NG, Burkert WL. Urokinase infusion for severe intraventricular hemorrhage. Acta Neurochir (Wien). 1995. 134: 55-9
32. Shimizu Y, Tsuchiya K, Fujisawa H. Endoscopic surgery for thalamic hemorrhage with intraventricular hemorrhage: Effects of combining evacuation of a thalamic hematoma to external ventricular drainage. Asian J Neurosurg. 2019. 14: 1112-5
33. Toyooka T, Kageyama H, Tsuzuki N, Ishihara S, Oka K. Flexible endoscopic aspiration for intraventricular casting hematoma. Acta Neurochir Suppl (Wien). 2016. 123: 17-23
34. Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Validation and comparison of models predicting survival following intracerebral hemorrhage. Crit Care Med. 1995. 23: 950-4
35. Yasargil MG, Yonekawa Y, Zumstein B, Stahl HJ. Hydrocephalus following spontaneous subarachnoid hemorrhage. Clinical features and treatment. J Neurosurg. 1973. 39: 474-9
36. Zhang Z, Li X, Liu Y, Shao Y, Xu S, Yang Y. Application of neuroendoscopy in the treatment of intraventricular hemorrhage. Cerebrovasc Dis. 2007. 24: 91-6
37. Zhou H, Cha Z, Wang L, Chen M, Zhang Q, Tang J. Clinical efficacy and safety of neuroendoscopic surgery for severe thalamic hemorrhage with ventricle encroachment. Neurosurg Rev. 2022. 45: 2701-8