- Department of Anaesthesia, Burjeel Royal Hospital, Al Ain, Abhudhabi, United Arab Emirates,
- Department of Anaesthesia and Intensive Care, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
- Department of Neurosurgery, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
Correspondence Address:
Dr Nidhi Bidyut Panda, Professor Neuroanaesthesia, Department of Anaesthesia and Intensive Care, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
DOI:10.25259/SNI_646_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Srikanth Koyanna1, Nidhi Bidyut Panda2, Shalvi Mahajan2, Neerja Bharti2, Swati Patel2, Navneet Singla3. The predictors of hyperglycemia and its effects on neurological outcome in the patients with aneurysmal subarachnoid hemorrhage undergoing surgical clipping: A prospective observational study. 14-Oct-2022;13:471
How to cite this URL: Srikanth Koyanna1, Nidhi Bidyut Panda2, Shalvi Mahajan2, Neerja Bharti2, Swati Patel2, Navneet Singla3. The predictors of hyperglycemia and its effects on neurological outcome in the patients with aneurysmal subarachnoid hemorrhage undergoing surgical clipping: A prospective observational study. 14-Oct-2022;13:471. Available from: https://surgicalneurologyint.com/surgicalint-articles/11928/
Abstract
Background: Following intracranial aneurysm rupture, 70–90% of patients have hyperglycemia as a stressful response. Uncontrolled hyperglycemia is deleterious if not controlled well. The objectives of the study were to assess the prevalence, risk factors of hyperglycemia, and its effect on outcome in aneurysmal subarachnoid hemorrhage (aSAH) patients who underwent aneurysmal clipping.
Methods: Following intracranial aneurysm rupture, 70–90% of patients have hyperglycemia as a stressful response. Uncontrolled hyperglycemia is deleterious if not controlled well. The objectives of the study were to assess the prevalence, risk factors of hyperglycemia, and its effect on outcome in aSAH patients who underwent aneurysmal clipping.
Results: At admission, the prevalence of hyperglycemia and severe hyperglycemia was 31.8% and 6.8%, respectively. Perioperative hyperglycemia and severe hyperglycemia were seen in 75.7% and 27%, respectively. History of diabetes mellitus (DM), higher admission random blood sugar, and higher admission mean blood pressure were predictors of perioperative hyperglycemia (P- 0.046, 0.00, and 0.004, respectively) and severe hyperglycemia (P- 0.048, 0.00, and 0.031). In addition, female sex, prolonged duration of anesthesia, and surgery were also found to be the predictors of hyperglycemia (P- 0.025, 0.07, and 0.012). Increased ventilator, intensive care unit, and hospital days were associated with perioperative hyperglycemia and severe hyperglycemia, respectively (P ≤ 0.006/0.00, P ≤ 0.007/0.00, and P ≤ 0.038/0.00). Poor Glasgow Outcome Score at 1 and 3 months after discharge was associated with admission and perioperative hyperglycemia ([P ≤ 0.000/0.000 and P ≤ 0.000/0.000], respectively). However, no association was seen between mortality and hyperglycemia or severe hyperglycemia.
Conclusion: A higher prevalence of hyperglycemia is present in aSAH patients. A higher incidence of perioperative hyperglycemia is associated with poor neurological outcomes. Hence, the identification of risk factors and meticulous perioperative control of hyperglycemia will help in preventing poor neurological outcomes.
Keywords: Aneurysmal subarachnoid hemorrhage, Hyperglycemia, Glasgow Outcome Scale
INTRODUCTION
Subarachnoid hemorrhage (SAH) is a terrifying condition.[
Approximately 75% of the patients have hyperglycemia and their blood glucose level exceeds 126–144 mg/dl during the first 2 weeks after aSAH.[
General anesthesia during aneurysmal clipping surgery further adds to the stress and can worsen hyperglycemia. For adequate glycemic control, it is important to know the predictors of hyperglycemia in this subset of patients. Very few studies have discussed predictors of hyperglycemia in aSAH.[
MATERIALS AND METHODS
A prospective, observational, and cohort study was conducted over a period of 1½years in a tertiary care institute in India. After obtaining clearance from the Institute Ethical Committee (NK/1341/MD/227, February 25, 2014) and informed written consent from the next of the kin of the patient, 150 subsequent adult patients (age ≥18 years) with the aSAH posted for clipping of aneurysm were included in the study.
Anesthesia protocol
All patients underwent a detailed preoperative evaluation before the surgery. Demographic data, preoperative blood glucose level, and vital parameters such as heart rate and mean blood pressure (MBP) at admission were noted. The previous history of DM and steroid intake before surgery was recorded. A standard anesthesia technique according to institute protocol was followed. The patients were induced with propofol (1–2 mg/kg) and intubated after vecuronium (0.1 mg/kg). Anesthesia was maintained with oxygen/nitrous oxide/propofol infusion and intermittent doses of vecuronium. Intraoperative analgesia was achieved with fentanyl (2 µg/kg) bolus followed by infusion at a rate of 2 µg/kg/h. In addition, to standard monitoring (electrocardiography, invasive arterial pressure, oxygen saturation, temperature, and end-tidal carbon dioxide), arterial blood gas analysis, blood sugar, electrolytes, and urine output were monitored. Hyperosmolar agent (mannitol 0.5 g/kg) was given to all patients. Patients received 0.9% saline as the intraoperative fluid. Target blood pressure was kept within 20% of the baseline value. Target end-tidal carbon dioxide was maintained between 32 and 38 mmHg depending on the brain condition.
Postoperatively, all patients were electively ventilated if required in the neurosurgical intensive care unit (ICU) for 12–24 h as per the institutional protocol. Patients were thoroughly assessed for blood sugar levels during the perioperative period. Intraoperative blood glucose was assessed hourly and the highest reading in the intraoperative period was recorded. Maximum blood glucose level per day in the postoperative period was noted during the hospital stay. Patients were divided into three groups based on random blood sugar (RBS): normoglycemia (≤160 mg/dl), hyperglycemia (>160 mg/dl), and severe hyperglycemia (>200 mg/dl).
All patients were followed up during their hospital stay for ventilator days, ICU days, and hospital days. In addition, major complications such as cerebral vasospasm, hydrocephalus, infection (pneumonia), and mortality were noted. After discharge from the hospital, patients were followed up at 30–90 days telephonically to assess neurological outcome using Glasgow Outcome Scale (GOS)[
Statistical analysis
All statistical analyses were performed using IBM SPSS (version 22), SPSS, Chicago, IL, and StatXact 3, Cytel Software, Cambridge, MA). Mean, standard deviation, and median with interquartile range were used to describe parametric data, whereas nonparametric data were stated as number (percentage). The independent sample t-test was used to compare parametric data between the two groups, while the Chi-square test was used to examine nonparametric data between the two groups. Using multivariate logistic regression, we created a multivariable model for independent predictors of hyperglycemia and severe hyperglycemia using candidate demographic and admission factors from the univariate study. Odds ratios within 95% confidence intervals were obtained. An independent sample t-test was used to determine the influence of hyperglycemia on the mean length of mechanical ventilation, ICU and hospital stay, and discharge GCS. We assessed the impact of perioperative hyperglycemia on mortality and GOS using multivariate logistic regression and odds ratios with 95% confidence intervals. For all analyses, significance was fixed at P ≤ 0.05 level.
RESULTS
A total of 150 patients of either sex presenting with cerebral aneurysm for intracranial clipping were enrolled in the study, of which two patients were excluded due to nonavailability of complete data. Hence, statistical analysis was carried out in 148 patients [
The demographical, baseline, and intraoperative parameters are described in
In our study, during the perioperative period, normoglycemia was seen in 36 (24.3%) patients, hyperglycemia was observed as 102 (75.7%) patients, and severe hyperglycemia was present in 10 (27%) patients. At least one episode of hyperglycemia was seen at admission, during intraoperative and postoperative period in 31.8%, 23%, and 73% of patients, respectively. Similarly, 6.8%, 6.1%, and 25% of patients had at least one episode of severe hyperglycemia at admission, during intraoperative and postoperative period, respectively.
In our study cohort, seven patients had a history of DM and all of them had perioperative hyperglycemia. Hence, the prevalence of hyperglycemia in a known diabetic patient was 100%. Out of seven patients, three patients had severe hyperglycemia at admission and five patients had shown severe hyperglycemia at least once during perioperative period. Therefore, the prevalence of severe hyperglycemia in a known diabetic patient was 42.85% during the preoperative period and 71.14% both during intraoperative and postoperative period.
The predictors of hyperglycemia and severe hyperglycemia during the perioperative period were determined using univariate analysis and are shown in
The parameters which were found to be significant by the Chi-square test and an independent sample t-test during univariate analysis were subjected to multivariate logistic regression and are presented in
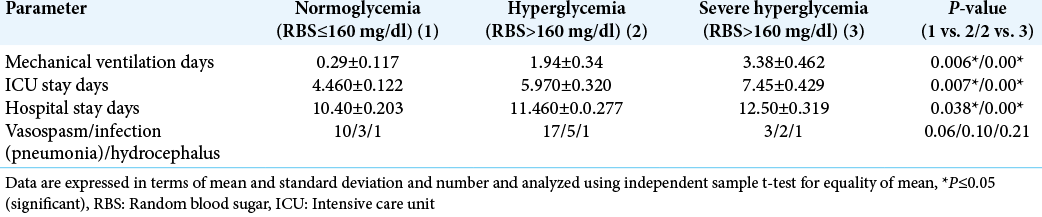
In this study, significantly increased duration of mechanical ventilation, ICU stay, and hospital stay was observed in patients with hyperglycemia and severe hyperglycemia compared to patients with normoglycemia (P < 0.05) [
Poor neurological outcome (GOS-1–3) at 30–90 days was seen in 58.8% and 70% of patients with hyperglycemia, 29.46% and 47.5% of patients with perioperative severe hyperglycemia, respectively (P < 0.05) [
DISCUSSION
In this study, the prevalence of hyperglycemia (RBS >160 mg/dl) and severe hyperglycemia (RBS >200 mg/dl) was 75.7% and 27%, respectively, in the perioperative period in patients with aSAH undergoing clipping surgery. All known diabetic patients on medication had shown hyperglycemia in the perioperative period (100%). However, the incidence of severe hyperglycemia in diabetic patients at admission was 42.85% and both during the intraoperative/postoperative period were 71.14%. This necessitates strict monitoring and management of hyperglycemia in all aSAH patients.
McGirt et al. found that 36% of patients had at least a single episode of hyperglycemia (RBS >200 mg/dl) in aSAH patients who underwent surgical or endovascular treatment.[
Aneurysmal SAH (aSAH), a stressful condition, causes activation of both the hypothalamic-pituitary-adrenal axis (HPA) and the sympathetic nervous system, leading to an increase in the levels of stress hormones. Stress hormones such as cortisol, growth hormone, and catecholamines further enhance glycogenolysis, gluconeogenesis, proteolysis, and lipolysis, and eventually leading to excessive glucose production. Furthermore, catecholamines are responsible for the development of insulin resistance and thereby augmenting glucose levels. Moreover, aSAH is accompanied by an increased inflammatory response and resultant cytokine release further enhances hyperglycemia and insulin resistance.[
Aneurysmal SAH (aSAH), a stressful condition, activates both the HPA and sympathetic nervous system. This initiates stress hormone production in the body. Stress hormones include cortisol, growth hormone, and catecholamines augment glycogenolysis, gluconeogenesis, proteolysis, and lipolysis, henceforth, excessive glucose production. Furthermore, development of insulin resistance with catecholamines also elevates blood glucose levels. An enhanced inflammatory response, and the cytokine release following aSAH, adds to hyperglycemia and insulin resistance.[
In our study, we evaluated predictors of hyperglycemia at admission and during the perioperative period in this subset of the population. On univariate analysis, female sex, high MBP, history of DM and high WFNS score at admission, high APACHE II score, longer duration of surgery, and anesthesia were significantly associated with perioperative hyperglycemia and severe hyperglycemia. Following multivariate logistic regression analysis, only three factors retained significance – high MBP, high RBS at admission, and high APACHE II score as the predictors of hyperglycemia/severe hyperglycemia.
Frontera et al. identified age ≥54 years, history of DM, high APACHE II score, and high Hunt-Hess grade as independent predictors of glucose burden (104 mg/dl) patients admitted to ICU with aSAH.[
Hyperglycemia was considered as a risk factor for an increase in ICU stay. In our study, we observed significantly longer duration of ventilator days, ICU days, and hospital days in patients with hyperglycemia (RBS >160 mg/dl) and severe hyperglycemia (RBS >200 mg/dl) when compared to normoglycemic groups.
Similarly, Badjatia et al. observed that hyperglycemia in aSAH patients who were admitted within 48 h of ictus was associated with longer ICU stay (14.5 ± 7.1 days vs. 11.6 ± 5.4 days; P < 0.001) and poor outcome at discharge (using modified Rankin score ≥3) in 58.9% versus 18.8% of patients (P < 0.001) compared to the normoglycemic group.[
We observed poor outcome (GOS-1–3) in patients who had hyperglycemia and severe hyperglycemia at admission or during the perioperative period but could not identify hyperglycemia as a predictor of mortality. Lanzino et al. were one of the first groups to identify an association between hyperglycemia and poor outcome in patients with aSAH.[
Rodriguez et al. found hyperglycemia with serum glucose >7.0 mmol/L as an independent predictor of mortality in patients with aSAH with rebleeding. The patient population selected accounts for difference in results as aSAH with rebleed comprises critical patients with a higher incidence of mortality (80%).[
In the present study, however, we found that hyperglycemia and severe hyperglycemia during the perioperative phase are related with a poor neurological outcome (GOS-1–3), but not with mortality. It may be due to protocolized management of hyperglycemia in ICU. Furthermore, hyperglycemia increases secondary brain injury by increasing matrix metalloproteinase activity, intravascular coagulation issues, and metabolic dysfunction. Consequently, even after hyperglycemia treatment, these patients are at a greater risk of having a poor neurological outcome.[
Limitations
All grades of SAH were not represented in our study. Therefore, the effect of hyperglycemia on outcome in all grades of aSAH could not be evaluated. We included only patients who came for aneurysmal clipping surgery and we have not included critical patients of aSAH managed conservatively. We did not include patients undergoing endovascular coiling of aneurysm which could have shown different relation of hyperglycemia with the outcome. Continuous blood sugar monitoring was not done, therefore, duration of hyperglycemia and severe hyperglycemia was not known. We did not measure cerebral glucose level; hence, we do not know actual cerebral glucose level during periods of hyperglycemia or severe hyperglycemia.
CONCLUSION
In patients with aSAH for clipping, the prevalence of perioperative hyperglycemia (RBS >160 mg/dl) was 75.7% and the prevalence of perioperative severe hyperglycemia (RBS >200 mg/dl) was 27%. History of DM, high RBS, and high MBP at admission was found to be the predictors of perioperative hyperglycemia and severe hyperglycemia. Hyperglycemia and severe hyperglycemia were associated with long duration of ventilator days, ICU days, hospital days, and poor GOS at 30–90 days after discharge. However, hyperglycemia and severe hyperglycemia were not associated with increase mortality in the hospital or at 30–90 days after discharge.
List of drugs used
Propofol Fentanyl Vecuronium Phenytoin
Ethics approval and consent to participate
Clearance to conduct the study was obtained from the Institute Ethics Committee in accordance with the Helsinki Declaration of 1975, as revised in 2000. Written informed consent was obtained from either patients or next of the kin all patients who participated in the study.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
References
1. Badjatia N, Topcuoglu MA, Buonanno FS, Smith EE, Nogueira RG, Rordorf GA. Relationship between hyperglycemia and symptomatic vasospasm after subarachnoid hemorrhage. Crit Care Med. 2005. 33: 1603-9
2. Bian L, Liu L, Wang C, Hussain M, Yuan Y, Liu G. Hyperglycemia within day 14 of aneurysmal subarachnoid hemorrhage predicts 1-year mortality. Clin Neurol Neurosurg. 2013. 115: 959-64
3. Dorhout Mees SM, van Dijk GW, Algra A, Kempink DR, Rinkel GJ. Glucose levels and outcome after subarachnoid hemorrhage. Neurology. 2003. 61: 1132-3
4. Frontera JA, Fernandez A, Claassen J, Schmidt M, Schumacher HC, Wartenberg K. Hyperglycemia after SAH: Predictors, associated complications, and impact on outcome. Stroke. 2006. 37: 199-203
5. Ingall TJ, Wiebers DO, Whisnant JP, editors. Natural history of subarachnoid hemorrhage. Stroke: Populations Cohorts, and Clinical Trials. Boston: Butterworth-Heinemann Ltd; 1993. p. 174-1864
6. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975. 1: 480-4
7. Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation afterfocal cerebral ischemia/reperfusion in rats: Relation to bloodbrain barrier dysfunction. Stroke. 2007. 38: 1044-9
8. Kruyt ND, Biessels GJ, de Haan RJ, Vermeulen M, Rinkel GJ, Coert B. Hyperglycemia and clinical outcome in aneurysmal subarachnoid hemorrhage: A meta-analysis. Stroke. 2009. 40: e424-30
9. Kruyt ND, Biessels GJ, DeVries JH, Luitse MJ, Vermeulen M, Rinkel GJ. Hyperglycemia in aneurysmal subarachnoid hemorrhage: A potentially modifiable risk factor for poor outcome. J Cereb Blood Flow Metab. 2010. 30: 1577-87
10. Lanzino G, Kassell NF, Germanson T, Truskowski L, Alves W. Plasma glucose levels and outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 1993. 79: 885-91
11. Maher BM, Jehangeer S, Majid HR. Hyperglycemia in acute subarachnoid hemorrhage. Pak J Neurol Sci. 2018. 13: 1-6
12. McGirt MJ, Woodworth GF, Ali M, Than KD, Tamargo RJ, Clatterbuck RE. Persistent perioperative hyperglycemia as an independent predictor of poor outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007. 107: 1080-5
13. Mocco J, Ransom ER, Komotar RJ, Schmidt JM, Sciacca RR, Mayer SA. Preoperative prediction of long-term outcome in poor-grade aneurysmal subarachnoid hemorrhage. Neurosurgery. 2006. 59: 529-38
14. Rivero Rodríguez D, Scherle Matamoros C, Fernández Cúe L, Miranda Hernández JL, Pernas Sánchez Y, Pérez Nellar J. Factors associated with poor outcome for aneurysmal subarachnoid haemorrhage in a series of 334 patients. Neurologia. 2017. 32: 15-21
15. Rodríguez DR, Matamoros CS, Cúe LF, Hernández JL, Sánchez YP, Nellar JP. Predictor’s of mortality in patients with aneurysmal subarachnoid hemorrhage and reebleding. Neurol Res Int. 2015. 2015: 545407
16. Schlenk F, Nagel A, Graetz D, Sarrafzadeh AS. Hyperglycemia and cerebral glucose in aneurysmal subarachnoid hemorrhage. Intensive Care Med. 2008. 34: 1200-7
17. Suarez JI. Does hyperglycemia contribute to secondary injury in subarachnoid hemorrhage?. Stroke. 2006. 37: 8-9
18. Van Gijin J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007. 369: 306-18