- Department of Neurosurgery, Nagoya University, Nagoya, Japan
- Department of Neurosurgery, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, Nagoya, Japan
- Department of Neurosurgery, Kariya Toyota General Hospital, Kariya, Japan.
Correspondence Address:
Yoshio Araki, Department of Neurosurgery, Nagoya University, Nagoya, Japan.
DOI:10.25259/SNI_772_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yoshio Araki1, Kinya Yokoyama1, Kenji Uda1, Fumiaki Kanamori1, Takashi Mamiya1, Kai Takayanagi1, Kazuki Ishii1, Kazunori Shintai2, Masahiro Nishihori1, Tetsuya Tsukada2, Kazuhito Takeuchi1, Kuniaki Tanahashi1, Yuichi Nagata1, Yusuke Nishimura1, Takafumi Tanei1, Yoshitaka Nagashima1, Shinsuke Muraoka3, Takashi Izumi1, Yukio Seki2, Ryuta Saito1. The preoperative focal cerebral blood flow status may be associated with slow flow in the bypass graft after combined surgery for moyamoya disease. 04-Nov-2022;13:511
How to cite this URL: Yoshio Araki1, Kinya Yokoyama1, Kenji Uda1, Fumiaki Kanamori1, Takashi Mamiya1, Kai Takayanagi1, Kazuki Ishii1, Kazunori Shintai2, Masahiro Nishihori1, Tetsuya Tsukada2, Kazuhito Takeuchi1, Kuniaki Tanahashi1, Yuichi Nagata1, Yusuke Nishimura1, Takafumi Tanei1, Yoshitaka Nagashima1, Shinsuke Muraoka3, Takashi Izumi1, Yukio Seki2, Ryuta Saito1. The preoperative focal cerebral blood flow status may be associated with slow flow in the bypass graft after combined surgery for moyamoya disease. 04-Nov-2022;13:511. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11974
Abstract
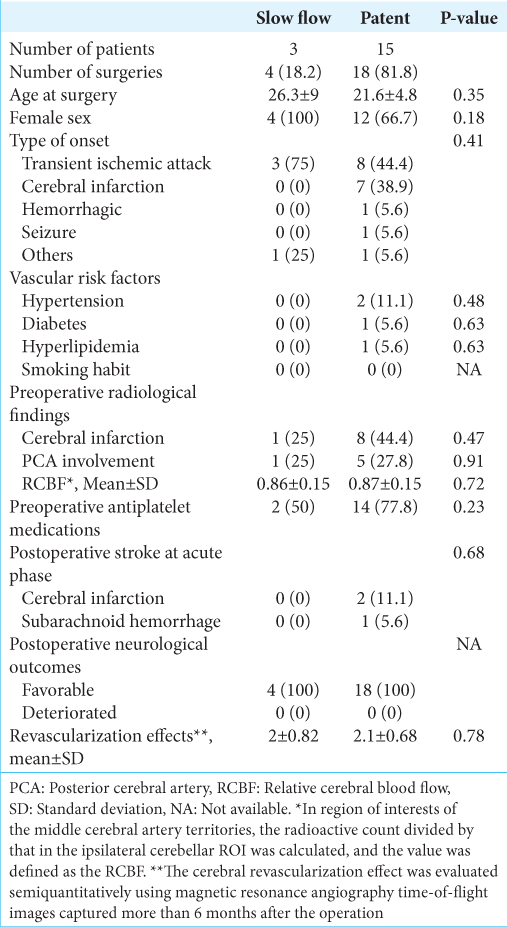
Background: The aim of this study was to investigate the association between early postoperative slow flow in bypass grafts and preoperative focal cerebral blood flow (CBF) in patients who underwent combined surgery for moyamoya disease (MMD).
Methods: The subjects were 18 patients (22 surgeries) who underwent single photon emission computed tomography (SPECT) before surgery. The CBF value of the middle cerebral artery territory was extracted from the SPECT data, and the value relative to the ipsilateral cerebellar CBF (relative CBF, or RCBF) was calculated. The association between RCBF and early postoperative slow flow in the bypass graft was investigated. In addition, the correlation between the revascularization effect and preoperative RCBF was analyzed.
Results: In four of 22 surgeries (18.2%), slow flow in the bypass graft was identified in the early postoperative period. Preoperative RCBF in the slow flow and patent groups was 0.86 ± 0.15 and 0.87 ± 0.15, respectively, with no significant difference (P = 0.72). The signal intensity of four slow-flowed bypasses was improved in all cases on magnetic resonance angiography images captured during the chronic phase (mean of 3.3 months postoperatively). The revascularization scores were 2 ± 0.82 and 2.1 ± 0.68 in the slow flow and patent groups, respectively, and did not differ significantly (P = 0.78). A significant correlation was not observed between preoperative RCBF and the revascularization effect.
Conclusion: No significant association was observed between preoperative RCBF and early postoperative slow flow in bypass grafts in patients with MMD undergoing combined surgery. Given the high rate of improved depiction of slow-flowed bypass in the chronic postoperative phase, the conceptual significance of an opportune surgical intervention is to maintain CBF by supporting the patient’s own intracranial-extracranial conversion function.
Keywords: Cerebral blood flow, Combined revascularization, Moyamoya disease, Recanalization, Slow-flowed direct bypass
INTRODUCTION
Moyamoya disease (MMD) is a rare disease with slowly progressive stenosis or occlusion at the end of the bilateral internal carotid artery (ICA). It is characterized by the development of penetrating branches at the bottom of the brain as collateral circulation.[
The pressure gradient between the donor and the recipient and the radius of the conduit plays an important role in the patency of the bypass.[
Studies focused on preoperative CBF have not been conducted on the slow flow in the direct bypass graft of combined surgery in the postoperative acute phase, which may help elucidate the pathophysiology of this unique phenomenon.
MATERIALS AND METHODS
Study population
Eighteen patients with MMD (22 surgeries) who underwent direct and indirect combined revascularization at our hospital from December 2019 to November 2020 were included in this study. All patient information was retrospectively collected from medical records with the approval of the Bioethics Review Board of Nagoya University Hospital. MMD was diagnosed by MRA based on the diagnostic criteria from the Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis.[
Clinical information, including age at surgery, type of onset (transient ischemic attack [TIA], cerebral infarction, seizure, and others), vascular risk factors (hypertension, diabetes, hyperlipidemia, and smoking habit), and preoperative antiplatelet therapy, was investigated by performing a chart review. Neurological outcomes at postoperative discharge were investigated using the modified Rankin Scale (mRS) as an index. If the mRS score increased by 1 point or more compared with before surgery, it was judged to be deteriorated; otherwise, it was judged to be favorable. Preoperative and postoperative radiological evaluations are described below.
Preoperative radiological findings
Preoperative magnetic resonance imaging (MRI) diffusion-weighted imaging or fluid-attenuated inversion recovery images confirmed the presence of cerebral infarction. MRA images were also used to evaluate preoperative posterior cerebral artery (PCA) involvement.
Acquisition of SPECT data
Cerebral perfusion SPECT imaging was performed using a double-headed gamma camera (Symbia T or Symbia T6, Siemens Healthcare, Erlangen, Germany). In SPECT imaging, we used the autoradiographic method with N-isopropyl-p-123I-iodoamphetamine (123I-IMP) for adult patients and the Patlak plot method with 99mTc-ethyl cysteinate dimer (99mTcECD) for pediatric patients. Regional CBF was measured for each preset region of interest (ROI) using iSSP and NEURO FLEXER software (Nihon Medi-Physics, Tokyo, Japan). The anterior cerebral artery (ACA), MCA, and PCA territories were automatically defined as ROIs according to the software settings. We have previously reported on these methodologies.[
Definition of relative CBF (RCBF) before surgery
In each ROI of the ACA, MCA and PCA territories, the radioactive count was divided by that in the ipsilateral cerebellar ROI, and the quotient was defined as the RCBF.[
Surgical procedures
Cerebral revascularization was performed on patients with MMD according to the policies established in the treatment guidelines.[
Postoperative management protocol
All cases, in this study, were managed using a standardized postoperative protocol.[
Relationship between preoperative RCBF and slow-flowed bypass early after surgery
Early postoperative slow flow in the bypass graft was determined by CTA performed on POD 1 or MRA performed on POD 2. Then, the association between RCBF and early postoperative slow flow in the bypass graft was statistically investigated.
Improved bypass flow and revascularization effect evaluated using MRA in the postoperative chronic phase
At our facility, regular MRI and MRA follow-up will be performed every 2–6 months after surgery. The examination confirmed the occurrence of a new postoperative stroke and the patency of the bypass graft. If the flow of the bypass graft that was slowed in the postoperative acute phase was improved, it was confirmed by MRA performed in this postoperative chronic phase.
The cerebral revascularization effect was evaluated semiquantitatively using MRA-TOF images captured more than 6 months after the operation. Similar to the method previously proposed by our group, the degree of angiogenesis observed from the ECA system was scored on the TOF images of some slices.[
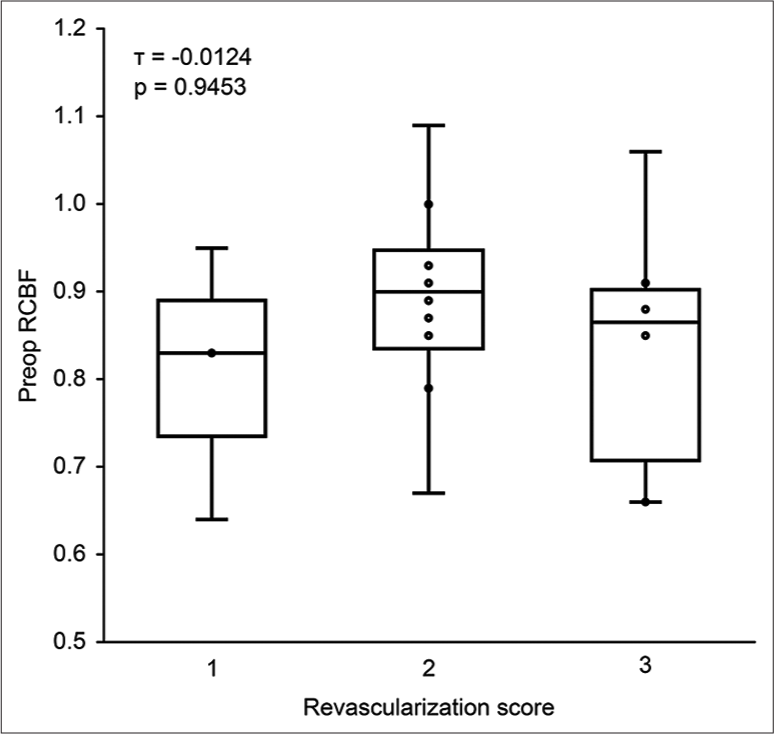
Correlation between preoperative RCBF and the effect of revascularization
The relationship between preoperative RCBF in the MCA territory where the recipient is expected to perfuse and the cerebral revascularization effect in the postoperative chronic phase was statistically investigated.
Statistical analysis
Normally distributed data are presented as the means ± standard deviations (SD). Non-normally distributed data are presented as the medians ± SD. In terms of the patient baseline characteristics, the type of onset and vascular risk factors was counted on a patient basis, while the other items were described on a hemisphere basis. RCBF in the preoperative MCA territory and revascularization effect in the group with slow-flowed bypass and the group with patent was compared using the Wilcoxon rank sum test. Kendall’s rank correlation coefficient (τ) was calculated to examine the correlation between the effect of cerebral revascularization measured on MRA images and preoperative RCBF.
JMP Pro version 15.1 (SAS Institute, Cary, NC) was used for statistical analyses. P < 0.05 indicated a significant difference.
RESULTS
The median age at surgery was 11 years (range 2–59 years). Fourteen of the 18 patients (77.8%) were female. In terms of onset, TIA accounted for half, followed by cerebral infarction in <30%. Only one case (5.6%) of hemorrhagic onset and seizure onset was identified. Regarding vascular risk factors, two patients presented hypertension (11.1%), and diabetes and hyperlipidemia were present in one patient (5.6%) each. The preoperative radiological examination revealed cerebral infarction in 40.9% and PCA involvement in 27.3% of the hemispheres. The calculated mean preoperative RCBF was 0.86 ± 0.14, which was approximately 15% lower than that of the ipsilateral cerebellum, which is considered normal CBF. Regarding the medication status of antiplatelet drugs, 72.7% used these drugs preoperatively. Direct and indirect combined surgery was performed in all cases for cerebral revascularization. Imaging examinations performed early after surgery confirmed patency (81.8%) in 18 of the 22 bypasses. Cerebral infarction was observed in two cases (9.1%), and subarachnoid hemorrhage (SAH) was observed in one case (4.5%) as early postoperative stroke events. On the other hand, no patients presented a decrease in the mRS score at the time of discharge of 1 point or more, and all patients had a favorable course.
The results of imaging examinations performed during the postoperative chronic phase showed that the flow of all four bypass grafts that had been slowed in the early postoperative period was improved (mean of 3.3 [range 2–5] months postoperatively). The mean value of the revascularization score evaluated by MRA more than 6 months after the operation was 2.1 ± 0.67, in which a moderate or higher revascularization effect had been obtained.
Comparative statistical analyses were performed between the slow flow group (four surgeries, 18.2%) and the patent group (18 surgeries, 81.8%) to investigate factors associated with slow flow in direct bypass grafts early after surgery [
Stroke events in the postoperative acute phase did not occur in the slow flow group. In the patent group, two cases of cerebral infarction (11.1%) and one case of SAH (5.6%) were observed, but the differences were not statistically significant (P = 0.68). The revascularization effects evaluated by MRA more than 6 months after the operation was 2 ± 0.82 for the slow flow group and 2.1 ± 0.68 for the patent group, showing no significant difference (P = 0.78).
Correlation between preoperative RCBF and the effect of revascularization
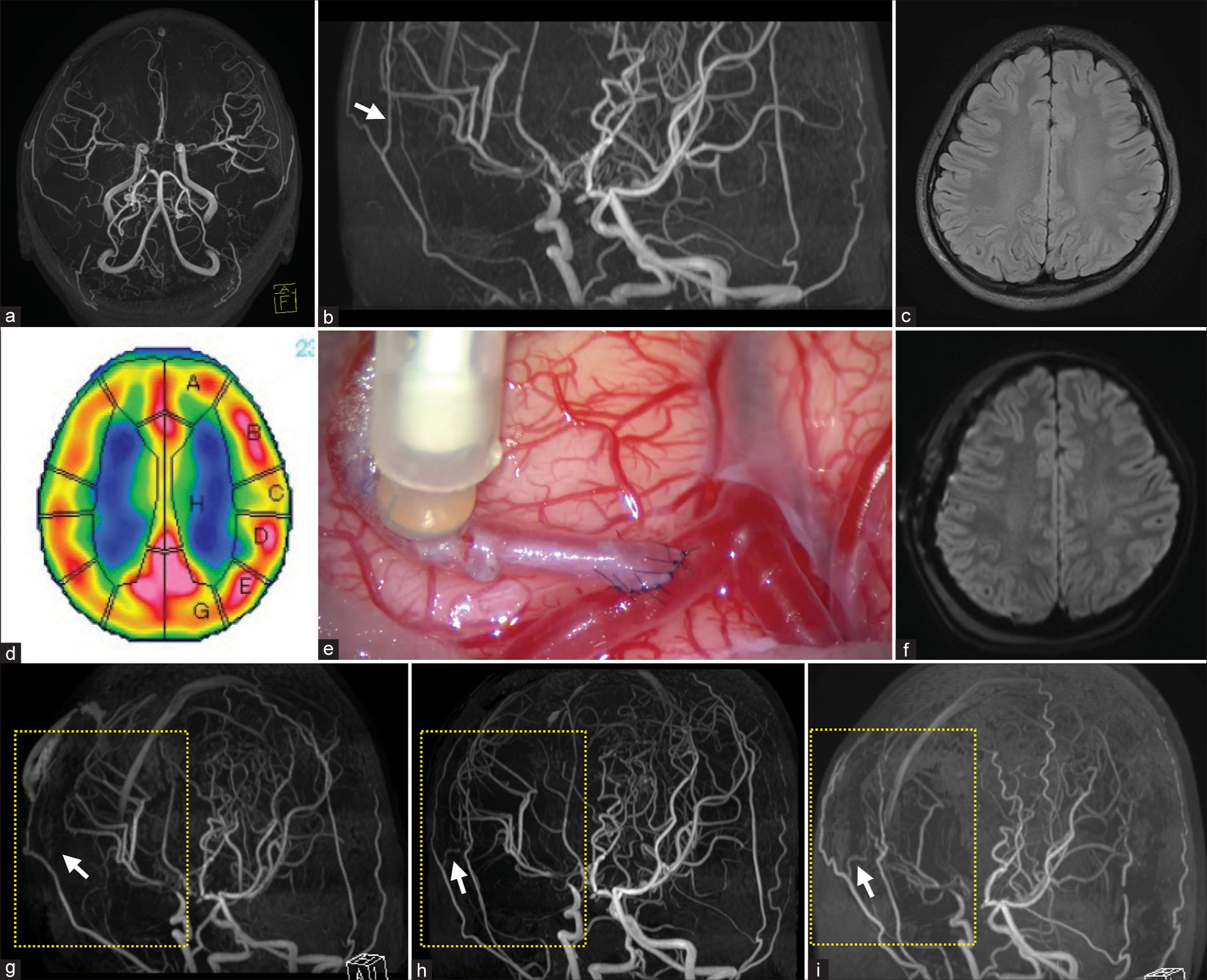
Illustrative case
An 11-year-old girl with transient motor weakness in the left upper limb was diagnosed with MMD and referred to our department for surgical treatment. Preoperative MRA showed bilateral ICA terminal stenosis, which was predominant on the right side. The development of MMVs was mild, and PCA involvement was not observed [
DISCUSSION
This study focused on cases, in which the flow of direct bypass, which was patent during surgery, was slowed early after surgery in patients who underwent direct and indirect combined surgery for MMD. As a result, no correlation was observed between preoperative RCBF, which is a focal CBF in the MCA territory, where the recipient is expected to perfuse, and slow flow of the bypass graft in the early postoperative period. However, in the subject analyzed in this study, the flow of all four slowed bypasses improved during the postoperative chronic phase [
Figure 2:
Illustrative case of moyamoya disease in an 11-year-old girl with postoperative improvement of flow in the direct bypass graft that was slowed early after combined surgery. Preoperative magnetic resonance angiography (MRA) images showed bilateral internal carotid artery terminal stenosis, which was predominant on the right side. The development of moyamoya vessels was mild, and no posterior cerebral artery involvement was observed (a). A developed superficial temporal artery (STA) parietal branch was observed on MRA images (b, white arrow). MRI did not reveal a preexisting cerebral infarction (c). In preoperative single photon emission computed tomography, the relative cerebral blood flow of the surrounding middle cerebral artery (MCA) territory perfused by the recipient candidate was 0.87. The region of interest indicates the perfusion area of the following vessels (A: anterior cerebral artery, B-D: MCA, E and G: posterior cerebral artery territory, H: perforators) (d). Doppler sonography and indocyanine green video angiography confirmed that the bypass immediately after the anastomosis was patent (e). No neurological abnormalities were observed after the operation, and no new cerebral infarction was found on the MRI diffusion weighted image captured on performed on postoperative day 2 (f). However, MRA showed that a flow of the bypass graft was not depicted (g, white arrow). Bypass grafts were visualized on the MRA images captured 2 months after the operation, suggesting that the flow was improved (h, white arrow). Afterward, the patient did not experience transient ischemic attack, and the graft was patent even on MRA images captured 25 months after the operation (i, white arrow). In MRA images from the early postoperative period to the chronic period, the depiction of the MCA donated by the internal carotid artery system decreased with the development of external carotid artery systems such as the STA and middle meningeal artery (g-i, yellow square dotted line).
This fact will complicate the estimation for patients with MMD, which is basically a bilateral disease. Similarly, the indirect revascularization effect that develops from the early postoperative period, which is peculiar to patients with MMD, makes it difficult to analyze the flow rate and pressure gradient by simply connecting the ECA system and the ICA system with a single graft.[
Therefore, we employed SPECT, which is among the most powerful tools available to quantitatively measure cerebral perfusion, to observe preoperative focal CBF in the MCA territory, where the recipient is expected to perfuse. Furthermore, the CBF was calculated not as a focal absolute value but as RCBF relative to the ipsilateral cerebellum, which is less susceptible to being influenced by crossed cerebellar diaschisis (CCD) and exhibits normal CBF.[
Although several studies have been conducted on early postoperative bypass occlusion with STA-MCA bypass for occlusive cerebrovascular disease due to cerebral atherosclerosis, quite few reports are available on early postoperative bypass occlusion for that procedure in patients with MMD. Takahashi and Yoshida[
On the other hand, in the MRA images captured in the postoperative chronic phase, improvement of flow of the bypass graft was obtained in all patients included in this series. Regarding the recanalization of a direct bypass once occluded, the study by Kim et al.[
Next, in combined revascularization, the effect of swelling of temporary muscle pedicles used for indirect bypass in the postoperative acute phase must be considered. Postoperative swelling of the temporalis muscle conveys potential risks of unpredictably narrowing the route through which the STA graft passes. This finding has been documented in reports from our own facility and those from other facilities where the swelling became symptomatic due to pressure on the brain.[
Numerous studies have been conducted on optimal surgical methods for MMD, but none have reached a conclusion.[
Limitations
First, since this study is a single-center retrospective study with a small number of patients, limited evidence is available to generalize the interpretation of the results to the patient population with MMD. A multicenter, prospective, and observational study is needed to obtain more convincing evidence. Second, ipsilateral cerebellar CBF, which is considered less susceptible to CCD, may not be interpreted as a normal value, depending on the target contralateral MMD stage. This conclusion is attributed to a decrease in CBF due to decreased metabolism caused by CCD, even if no significant stenosis of the vertebrobasilar artery is observed. Therefore, the MMD stage on the opposite side must be considered to improve the accuracy of CBF evaluation using RCBF. Third, if patients with severely reduced preoperative RCBF had been included, the results might have been different for the association between RCBF and early postoperative slow flow in the bypass graft and revascularization efficacy. However, the fact that most of the subjects in the present study (77.8%) had an ischemic onset reminds us that CBF is simply not directly associated with the development of neurological symptoms or stroke. A solution to this problem would be to enroll more cases and compare preoperative RCBF in cohorts matched for background factors such as onset type, age group, MMD disease stage, and imaging findings. Subsequent stratification of preoperative RCBF would ideally be used to analyze the effects on postoperative bypass patency and revascularization effects. Fourth, scoring with MRA TOF images used to assess the revascularization effect is limited by its dependence on MRA resolution and its semiquantitative aspect. Since revascularization occurs in the small arteries of the ECA system, evaluation by MRA, which visualizes blood flow velocity, may not be sufficient for analysis. Originally, it should be evaluated with fine angiographic images by DSA/ CTA. However, the method using DSA is similarly limited to semiquantitative evaluation.[
CONCLUSION
No significant association was observed between preoperative focal CBF and early postoperative slow-flowed bypass in patients with MMD undergoing combined surgery. Given the highly improved depiction of slow-flowed bypass in the chronic postoperative phase, maintaining CBF by supporting the patient’s own IC-EC conversion function is the conceptual significance of opportune surgical intervention.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Araki Y, Mamiya T, Fujita N, Uda K, Yokoyama K, Kanamori F. Changes in cerebral blood flow in the postoperative chronic phase after combined cerebral revascularization for moyamoya disease with ischaemic onset. Neurosurg Rev. 2022. 45: 2471-80
2. Araki Y, Uda K, Yokoyama K, Kanamori F, Kurimoto M, Shiba Y. Challenging direct bypass surgery for very young children with moyamoya disease: Technical notes. Neurosurg Rev. 2022. 45: 1799-807
3. Araki Y, Uda K, Yokoyama K, Kanamori F, Mamiya T, Nishihori M. Surgical designs of revascularization for moyamoya disease: 15 years of experience in a single center. World Neurosurg. 2020. 139: e325-34
4. Araki Y, Yokoyama K, Uda K, Kanamori F, Kurimoto M, Shiba Y. Postoperative stroke and neurological outcomes in the early phase after revascularization surgeries for moyamoya disease: An age-stratified comparative analysis. Neurosurg Rev. 2021. 44: 2785-95
5. Araki Y, Yokoyama K, Uda K, Kanamori F, Mamiya T, Nishihori M. Ipsilateral late stroke after revascularization surgery for patients with moyamoya disease. Acta Neurochir (Wien). 2021. 163: 1493-502
6. Deng X, Gao F, Zhang D, Zhang Y, Wang R, Wang S. Direct versus indirect bypasses for adult ischemic-type moyamoya disease: A propensity score-matched analysis. J Neurosurg. 2018. 128: 1785-91
7. Fujimura M, Kaneta T, Shimizu H, Tominaga T. Cerebral ischemia owing to compression of the brain by swollen temporal muscle used for encephalo-myo-synangiosis in moyamoya disease. Neurosurg Rev. 2009. 32: 245-9 discussion 249
8. Fujimura M, Tominaga T. Lessons learned from moyamoya disease: Outcome of direct/indirect revascularization surgery for 150 affected hemispheres. Neurol Med Chir (Tokyo). 2012. 52: 327-32
9. Fujimura M, Tominaga T, Kuroda S, Takahashi JC, Endo H, Ogasawara K. 2021 Japanese guidelines for the management of moyamoya disease: Guidelines from the research committee on moyamoya disease and Japan stroke Society. Neurol Med Chir (Tokyo). 2022. 62: 165-70
10. Hojo M, Hoshimaru M, Miyamoto S, Taki W, Nagata I, Asahi M. Role of transforming growth factor-beta1 in the pathogenesis of moyamoya disease. J Neurosurg. 1998. 89: 623-9
11. Hoshimaru M, Takahashi JA, Kikuchi H, Nagata I, Hatanaka M. Possible roles of basic fibroblast growth factor in the pathogenesis of moyamoya disease: An immunohistochemical study. J Neurosurg. 1991. 75: 267-70
12. Houkin K, Nakayama N, Kuroda S, Ishikawa T, Nonaka T. How does angiogenesis develop in pediatric moyamoya disease after surgery? A prospective study with MR angiography. Childs Nerv Syst. 2004. 20: 734-41
13. Jafar JJ, Russell SM, Woo HH. Treatment of giant intracranial aneurysms with saphenous vein extracranial-to-intracranial bypass grafting: Indications, operative technique, and results in 29 patients. Neurosurgery. 2002. 51: 138-44 discussion 144-36
14. Kanamori F, Araki Y, Yokoyama K, Uda K, Mamiya T, Nishihori M. Effects of aspirin and heparin treatment on perioperative outcomes in patients with moyamoya disease. Acta Neurochir (Wien). 2021. 163: 1485-91
15. Kanamori F, Araki Y, Yokoyama K, Uda K, Nishihori M, Izumi T. Brain compression by encephalo-myosynangiosis is a risk factor for transient neurological deficits after surgical revascularization in pediatric patients with moyamoya disease. World Neurosurg. 2020. 133: e558-66
16. Kim DS, Huh PW, Kim HS, Kim IS, Choi S, Mok JH. Surgical treatment of moyamoya disease in adults: Combined direct and indirect vs. indirect bypass surgery. Neurol Med Chir (Tokyo). 2012. 52: 333-8
17. Kim SH, Lee H, Yoo M, Jin S, Lee S, Choi BS. Angiographic and clinical outcomes of non-patent anastomosis after bypass surgery in adult moyamoya disease. Acta Neurochir (Wien). 2019. 161: 379-84
18. Kuroda S, Houkin K. Moyamoya disease: Current concepts and future perspectives. Lancet Neurol. 2008. 7: 1056-66
19. Kuroda S, Nakayama N, Yamamoto S, Kashiwazaki D, Uchino H, Saito H. Late (5-20 years) outcomes after STA-MCA anastomosis and encephalo-duro-myo-arteriopericranial synangiosis in patients with moyamoya disease. J Neurosurg. 2020. 134: 909-16
20. Maruwaka M, Yoshikawa K, Okamoto S, Araki Y, Sumitomo M, Kawamura A. Biomarker research for moyamoya disease in cerebrospinal fluid using surface-enhanced laser desorption/ionization time-of-flight mass spectrometry. J Stroke Cerebrovasc Dis. 2015. 24: 104-11
21. Matsushima T, Inoue T, Suzuki SO, Fujii K, Fukui M, Hasuo K. Surgical treatment of moyamoya disease in pediatric patients--Comparison between the results of indirect and direct revascularization procedures. Neurosurgery. 1992. 31: 401-5
22. Mikami T, Suzuki H, Ukai R, Komatsu K, Akiyama Y, Wanibuchi M. Predictive factors for acute thrombogenesis occurring immediately after bypass procedure for moyamoya disease. Neurosurg Rev. 2020. 43: 609-17
23. Miyamoto S, Yoshimoto T, Hashimoto N, Okada Y, Tsuji I, Tominaga T. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: Results of the Japan adult moyamoya trial. Stroke. 2014. 45: 1415-21
24. Muraoka S, Araki Y, Kondo G, Kurimoto M, Shiba Y, Uda K. Postoperative cerebral infarction risk factors and postoperative management of pediatric patients with moyamoya disease. World Neurosurg. 2018. 113: e190-9
25. Nanba R, Kuroda S, Ishikawa T, Houkin K, Iwasaki Y. Increased expression of hepatocyte growth factor in cerebrospinal fluid and intracranial artery in moyamoya disease. Stroke. 2004. 35: 2837-42
26. Rashad S, Saqr KM, Fujimura M, Niizuma K, Tominaga T. The hemodynamic complexities underlying transient ischemic attacks in early-stage moyamoya disease: An exploratory CFD study. Sci Rep. 2020. 10: 3700
27. Scharf J, Schmiedek P, Kemmling A, Gerigk L, Groden C, Horn P. Spontaneous recanalization of occluded standard extracranial-intracranial arterial bypass. Cerebrovasc Dis. 2007. 23: 175-80
28. Setta K, Kojima D, Shimada Y, Yoshida J, Oshida S, Fujimoto K. Accuracy of brain perfusion single-photon emission computed tomography for detecting misery perfusion in adult patients with symptomatic ischemic moyamoya disease. Ann Nuclear Med. 2018. 32: 611-9
29. Sia SF, Davidson AS, Assaad NN, Stoodley M, Morgan MK. Comparative patency between intracranial arterial pedicle and vein bypass surgery. Neurosurgery. 2011. 69: 308-14
30. Sia SF, Qian Y, Zhang Y, Morgan MK. Mean arterial pressure required for maintaining patency of extracranial-tointracranial bypass grafts: An investigation with computational hemodynamic models-case series. Neurosurgery. 2012. 71: 826-31
31. Sundt TM, Sundt TM. Principles of preparation of vein bypass grafts to maximize patency. J Neurosurg. 1987. 66: 172-80
32. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease, Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969. 20: 288-99
33. Takahashi S, Yoshida K. Delayed reopening of a superficial temporal artery to middle cerebral artery bypass graft occluded by a white thrombus during surgery. Surg Neurol Int. 2020. 11: 220
34. Tsukada T, Izumi T, Isoda H, Nishihori M, Kropp AE, Mizuno T. Comparison of hemodynamic stress in healthy vessels after parent artery occlusion and flow diverter stent treatment for internal carotid artery aneurysm. J Neurosurg. 2022. 136: 619-26
35. Uchino H, Kim JH, Fujima N, Kazumata K, Ito M, Nakayama N. Synergistic interactions between direct and indirect bypasses in combined procedures: The significance of indirect bypasses in moyamoya disease. Neurosurgery. 2017. 80: 201-9
36. Uda K, Araki Y, Muraoka S, Ota S, Wada K, Yokoyama K. Intraoperative evaluation of local cerebral hemodynamic change by indocyanine green videoangiography: Prediction of incidence and duration of postoperative transient neurological events in patients with moyamoya disease. J Neurosurg. 2018. 1: 1-9
37. Wang KC, Phi JH, Lee JY, Kim SK, Cho BK. Indirect revascularization surgery for moyamoya disease in children and its special considerations. Korean J Pediatr. 2012. 55: 408-13
38. Yokoyama K, Maruwaka M, Yoshikawa K, Araki Y, Okamoto S, Sumitomo M. Elevation of proenkephalin 143-183 in cerebrospinal fluid in moyamoya disease. World Neurosurg. 2018. 109: e446-59