- Department of Neurosurgery, Dr. Soeradji Tirtonegoro Central Public Hospital, Klaten, Indonesia
- Department of Neurosurgery, Faculty of Medicine, Mataram University/West Nusa Tenggara General Hospital, Mataram City Lombok Island, Indonesia
- Department of Neurosurgery, Faculty of Medicine, Udayana University/Prof. Dr. I Goesti Ngoerah Gde Ngoerah Hospital, Denpasar, Indonesia
- Department of Neurosurgery, Faculty of Medicine, Sebelas Maret University/Dr. Moewardi General Public Hospital, Surakarta, Indonesia.
Correspondence Address:
Hanan Anwar Rusidi, Department of Neurosurgery, Dr. Soeradji Tirtonegoro Central Public Hospital, Klaten, Indonesia.
DOI:10.25259/SNI_849_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hanan Anwar Rusidi1, Rohadi Muhammad Rosyidi2, Dewa Putu Wisnu Wardhana3, Wisnu Baskoro1, Geizar Arsika Ramadhana4. The role of preoperative hematological inflammatory markers as a predictor of meningioma grade: A systematic review and meta-analysis. 08-Mar-2024;15:77
How to cite this URL: Hanan Anwar Rusidi1, Rohadi Muhammad Rosyidi2, Dewa Putu Wisnu Wardhana3, Wisnu Baskoro1, Geizar Arsika Ramadhana4. The role of preoperative hematological inflammatory markers as a predictor of meningioma grade: A systematic review and meta-analysis. 08-Mar-2024;15:77. Available from: https://surgicalneurologyint.com/surgicalint-articles/12789/
Abstract
Background: Inflammatory processes play an important role in the aggressiveness of a tumor. However, the relationship between inflammatory markers in meningioma grade is not well known. Knowledge of preoperative meningioma grade plays an important role in the prognosis and treatment of this tumor. This study aims to assess preoperative hematological inflammatory markers as a predictor of the pathological grade of meningioma.
Methods: To ensure comprehensive retrieval of relevant studies, we searched the following key databases, PubMed, Science Direct, and Biomed Central, with evidence related to preoperative hematological inflammatory markers among meningioma up to September 2023. The studies involved were selected based on established eligibility criteria. The analysis in this study uses Review Manager 5.4
Results: Six studies were obtained from the search results. The total number of patients 2789 (469 high-grade meningioma and 2320 low-grade meningioma) analysis shows elevated neutrophil-to-lymphocyte ratio (NLR) (mean difference [MD]: 0.29; 95% confidence interval [CI] 0.13–0.45; P = 0.0004), monocyte-to-lymphocyte ratio (MLR) (MD: 0.02; 95% CI 0.00–0.04; P = 0.003), and low lymphocyte-to-monocyte ratio (LMR) (MD: −0.82; 95% CI −1.46–−0.18; P = 0.005) significantly associated with high-grade meningioma compared to low-grade meningioma. No significant correlation between high-grade and low-grade meningioma based on platelet-lymphocyte ratio value is observed.
Conclusion: The parameters of NLR, MLR, and LMR have been found to be cost-effective preoperative methods that demonstrate potential value in the prediction of meningioma grade. To enhance the reliability of the findings, it is imperative to do further prospective study.
Keywords: Grade, Inflammatory markers, Meningioma
INTRODUCTION
Meningioma ranks as the most common brain tumor in adults. In cases of central nervous system (CNS) tumors, 39.3% are meningiomas and account for 55.4% of benign CNS tumors.[
Meningiomas originate from meningothelial (arachnoid) cells on the inner surface of the dura.[
Surgical management with total removal is still the main choice in cases of high-grade meningioma. In high-grade meningioma, adjuvant therapy is usually required due to the high risk of recurrence and poor prognosis.[
Various hematological inflammatory markers are currently attracting the attention of researchers, including their association with tumors. Progression of a tumor is closely linked to the inflammatory process. Immune cells in the inflammatory process will infiltrate the tumor; this process can be seen from the peripheral blood hematology test.[
Regular blood tests performed before surgery are widely available and inexpensive. Therefore, predicting the grade of a tumor preoperatively using hematological indicators is promising. The previous meta-analyses have assessed hematological inflammatory parameters in meningioma progression and recurrence.[
MATERIALS AND METHODS
This study was carried out in compliance with the guidelines and criteria established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol.
Search strategy
We systematically searched PubMed, Science Direct, and Biomed Central databases from their inception till September 2023. We use two stages in article search. The first search uses keywords with Boolean operators (inflammatory markers AND meningioma). Then, for the second search, we used Boolean operators for several hematological inflammatory markers: neutrophil-lymphocyte ratio AND meningioma, platelet lymphocyte ratio AND meningioma, and monocyte lymphocyte ratio AND meningioma.
Eligibility criteria
To be eligible for incorporation, investigations should clearly specify and depict the research population, the treatments, and the outcome or prognosis. The study included: (1) patients were diagnosed with low-grade and high-grade and histologically verified and graded according to the WHO diagnostic criteria, (2) preoperative levels of systemic inflammatory markers were evaluated, and (3) information on outcome results was documented. Studies were excluded: (1) review articles, case reports, conference abstracts, and letters, (2) animal or cell studies, and (3) not in English language.
Data synthesis and extraction
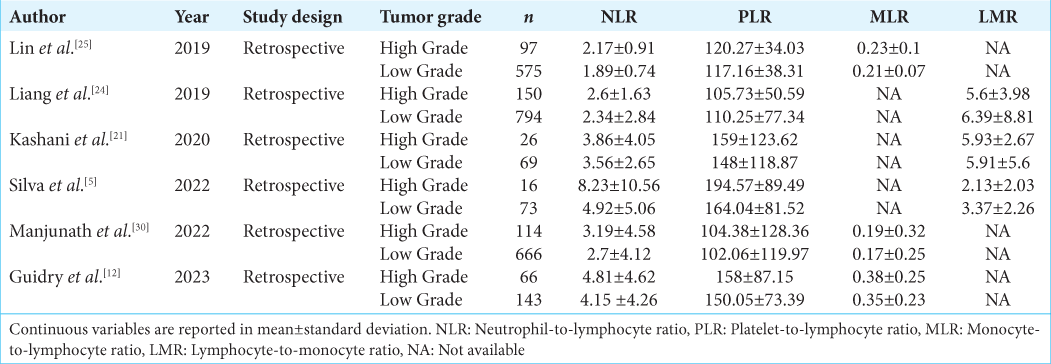
Data extracted from the identified publication included the first author, year published, study design, number of patients, preoperative hematology data, and results from each study, including tumor grade and mean and standard deviation (SD) values. The extracted data were displayed in tables and analyzed further [
Study quality assessment
The quality of the included papers was evaluated by two reviewers using the Joanna Briggs Institute (JBI) critical assessment techniques for cohort studies. To determine the overall quality of reporting in the study, a scoring system was devised, where each “yes” response received one point, while “no” and “unclear” responses received zero points. Subsequently, the total score was divided by the total number of questions on the checklist. The resulting score was then used to classify the study’s quality as high (<50%), moderate (50–69%), or indicating a low risk of bias (≥70%). This approach allowed for a conclusive assessment of the study’s reporting quality based on a systematic evaluation of various criteria outlined in the checklist.
Statistical analysis
We extracted continuous data as a mean from each selected study. The data obtained were then analyzed and presented in a forest plot. We used the random effect model in the analysis due to the possibility of different treatments between different and multiple studies. There were normally distributed and abnormally distributed data. Each demographic data was converted to mean and SD.
The I2 statistic was employed to assess the level of statistical heterogeneity among the studies. Heterogeneity was categorized based on the I2 value, with values below 50% indicating minimal to moderate heterogeneity and above 50% indicating substantial heterogeneity. Statistical analyses were conducted using Review Manager 5.4, which generated forest plots to summarize the meta-analysis results. Continuous variables were analyzed using the mean difference (MD). A significance level of P < 0.05 was set to determine statistical significance.
RESULTS
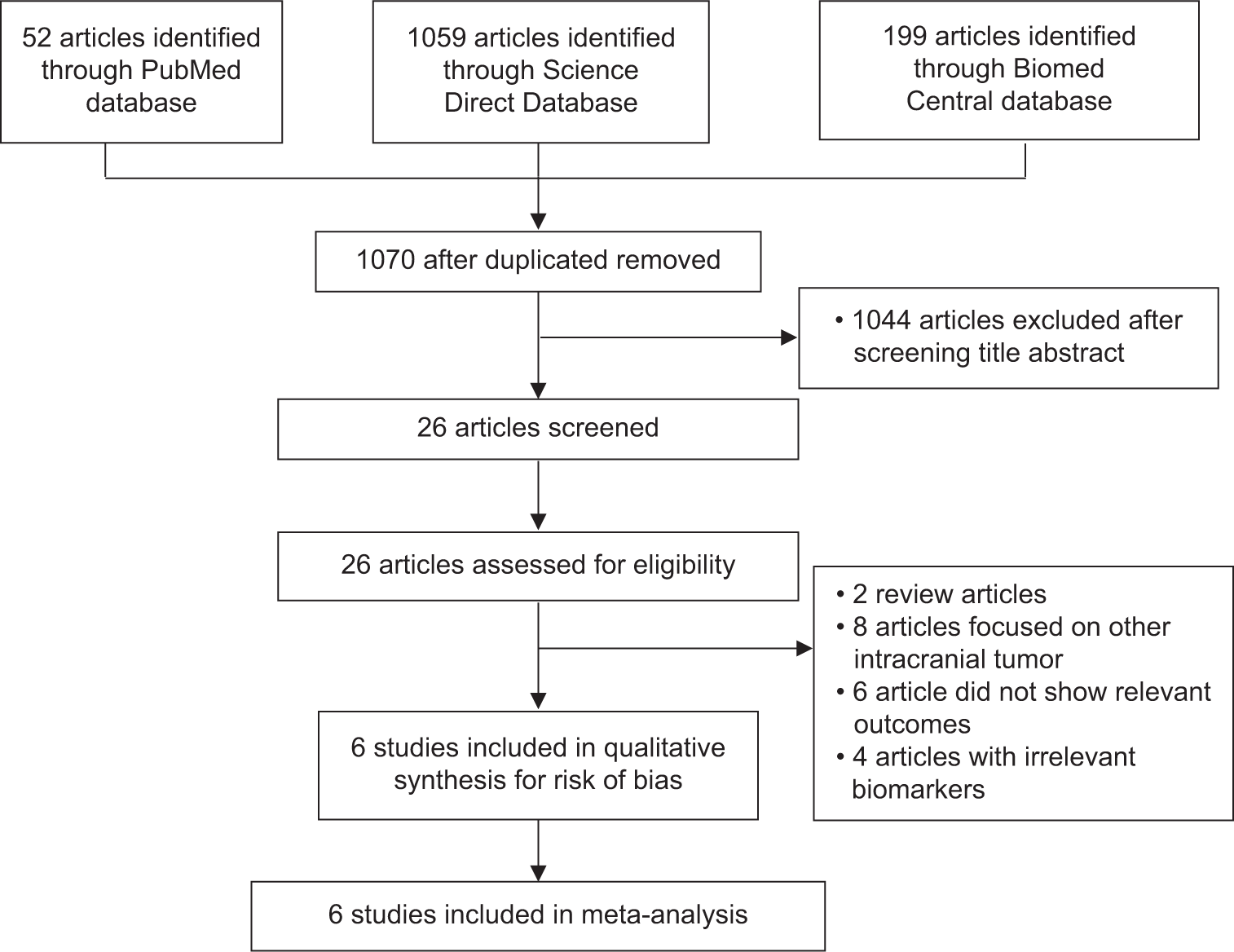
The flowchart for searching articles that fulfill the inclusion criteria for this study is shown in
A total of 1310 studies were obtained from the initial search of the database. After the duplicated papers were excluded, 1.070 articles were obtained. Then, screening was carried out based on the title and abstract. After screening, 26 articles were selected and then matched with eligibility criteria. After several stages of eligibility screening, a total of six studies were included in this study.[
We included six studies with retrospective design involving a minimum of 89 patients and a maximum of 944 patients for the meta-analysis. The total number of patients was 2789, with 469 high-grade meningioma patients and 2320 low-grade meningioma patients. The outcomes included in the meta-analysis are NLR, PLR, MLR, and LMR.
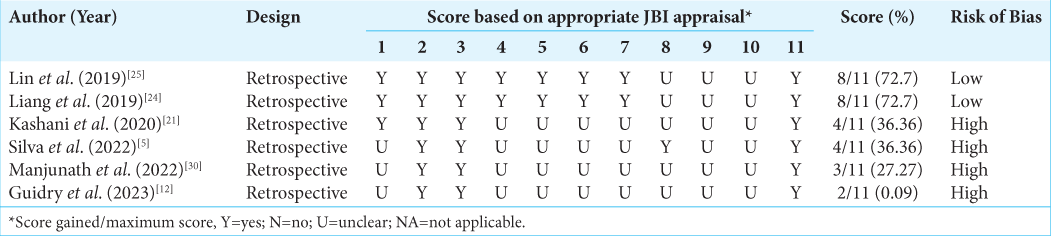
Quality of included studies
The quality assessment of the included observational studies using the JBI critical appraisal tool yielded varied results, which are summarized in
NLR
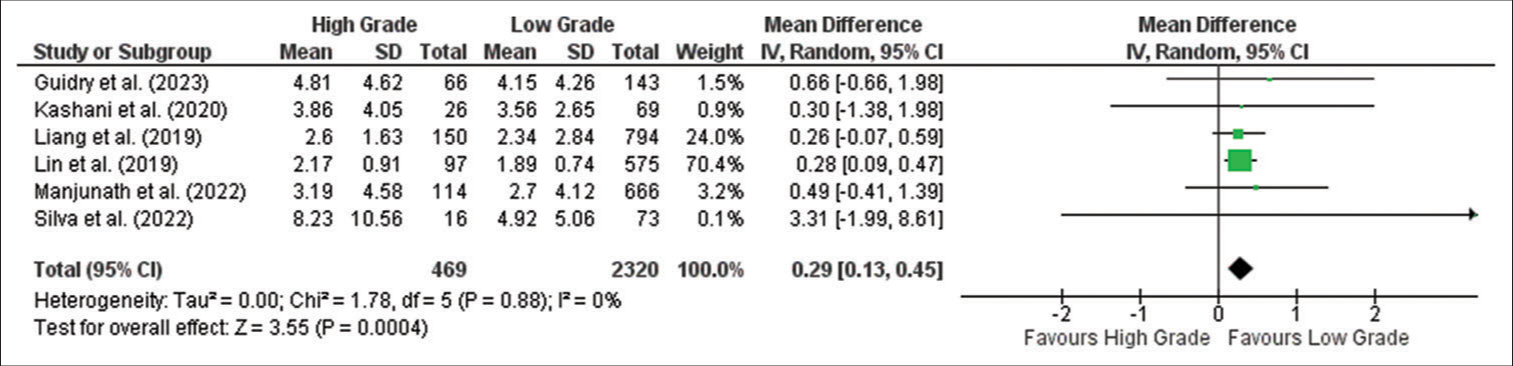
Meta-analysis showed that the NLR is significantly higher in the high grade compared to the low-grade tumor group (MD: 0.29; 95% confidence interval [CI] 0.13–0.45; P = 0.0004), as shown in the forest plot in
Figure 2:
Forest plot of neutrophil-to-lymphocyte ratio between high-grade and low-grade tumor groups. CI: Confidence interval, SD: Standard deviation. The green box shows the effect value of each study and the size indicates the weight of the study. The black rectangle shows the combined effect value of each study. The analysis showed that NLR was higher in high grade meningioma than in low grade meningioma significantly.
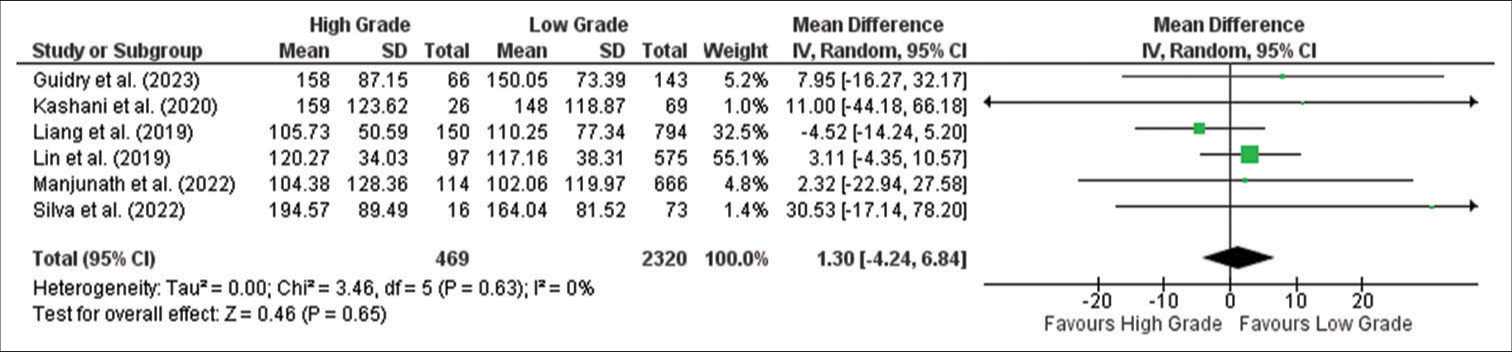
PLR
Meta-analysis showed no significant relationship between PLR values in high-grade and low-grade meningioma (MD: 1.30; 95% CI −4.24–6.84; P = 0.65), as shown in the forest plot in
Figure 3:
Forest plot of platelet-to-lymphocyte ratio between high-grade and low-grade tumor groups. CI: Confidence interval, SD: Standard deviation. The green box shows the effect value of each study and the size indicates the weight of the study. The black rectangle shows the combined effect value of each study. Analysis showed PLR has no significant relationship between high-grade and low-grade meningioma.
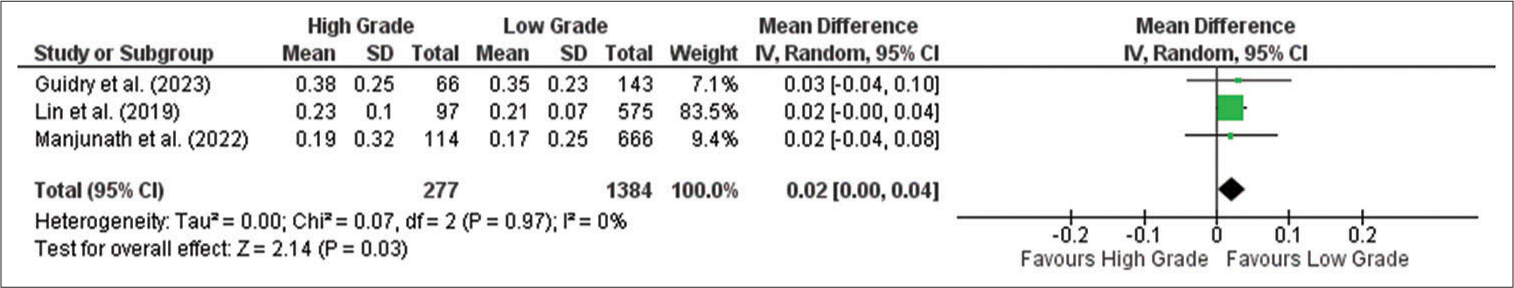
MLR
Meta-analysis showed that the MLR is significantly higher in the high-grade compared to the low-grade meningioma group (MD: 0.02; 95% CI 0.00–0.04; P = 0.003), as shown in the forest plot in
Figure 4:
Forest plot of monocyte-to-lymphocyte ratio between high-grade and low-grade tumor groups. CI: Confidence interval, SD: Standard deviation. The green box shows the effect value of each study and the size indicates the weight of the study.The black rectangle shows the combined effect value of each study. The analysis showed that MLR was higher in high grade meningioma than in low grade meningioma significantly.
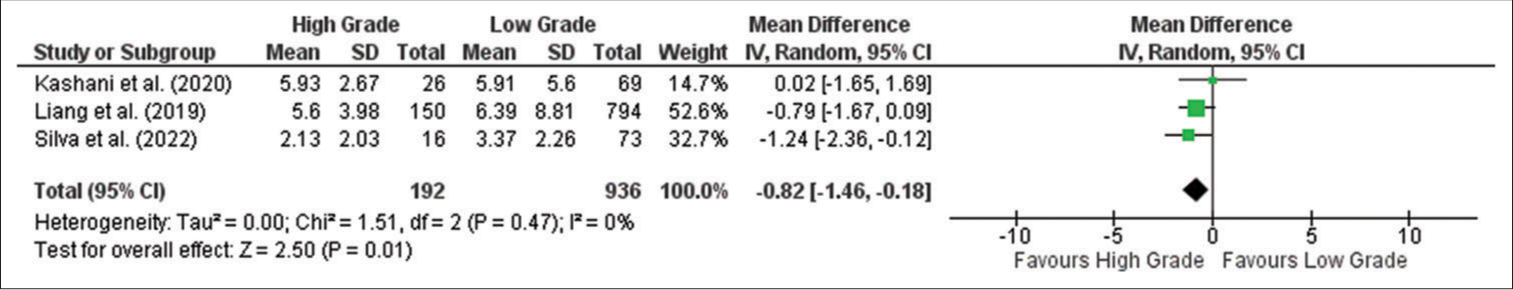
LMR
Meta-analysis showed that the LMR is significantly lower in the high-grade compared to the low-grade tumor group (MD: −0.82; 95% CI −1.46–−0.18; P = 0.005), as shown in the forest plot in
Figure 5:
Forest plot of lymphocyte-to-monocyte ratio between high-grade and low-grade tumor groups. CI: Confidence interval, SD: Standard deviation. The green box shows the effect value of each study and the size indicates the weight of the study. The black rectangle shows the combined effect value of each study. The analysis showed that LMR was lower in high grade meningioma than in low grade meningioma significantly.
DISCUSSION
Meta-analysis addressing the relationship between hematological inflammatory markers and meningioma grade has not been reported to our knowledge. This study found that higher NLR and MLR and lower LMR were associated with high-grade meningiomas. These hematological parameters may be able to predict tumor grade before surgery. This may help clinicians to plan treatment early, thereby improving patient prognosis. These markers are also derived from a routine complete blood count, which is readily available and provides a cost-effective method for preoperative assessment. Therefore, hematologic parameters that are predictive of high-grade meningiomas, which have a poorer prognosis, can be used to guide the decision for advanced treatment.
Six studies met the inclusion criteria and were included for meta-analysis to explore the role of inflammatory markers in predicting meningioma grade. Meningiomas, the most common primary brain tumors, display a spectrum of biological behaviors that range from benign to aggressive. Meningiomas are a type of brain tumor that can exhibit varying levels of aggressiveness. Understanding the factors that can predict tumor grade is crucial for accurate prognosis and treatment planning.
The quality assessment of the included studies varied, with most studies categorized as having a moderate or low risk of bias. In addition, heterogeneity between studies was observed in some analyses, which may be due to differences in patient characteristics and measurement methods. Nevertheless, this meta-analysis provides valuable insights into the potential role of preoperative hematological inflammatory markers in meningioma management and prognosis.
Tumor tissue infiltration is a condition that involves an inflammatory process. Tumor-associated macrophages in the tumor stroma induce immune cell responses and various cytokines.[
Neutrophils are the most abundant component of white blood cells in the circulation.[
Similar patterns were observed with MLR. This marker is significantly higher in the high grade compared to the low-grade meningioma group (MD: 0.02; 95% CI 0.00– 0.04; P = 0.003), reinforcing the link between tumor grade and these inflammatory markers. Meanwhile, the LMR parameter showed significantly lower results in high-grade meningioma compared to low-grade meningioma (MD: −0.82; 95% CI −1.46–−0.18; P = 0.005). Monocytes can easily infiltrate the blood−brain barrier (BBB) in cases of brain tumors.[
Furthermore, lymphocytes in the circulating tumor microenvironment will become tumor-infiltrating lymphocytes (TIL) and act as anti-tumor through cytolytic activity, antiproliferation, and inhibit migration.[
Platelets are associated with the formation of tumor cell-platelet aggregation that supports tumor cell growth and angiogenesis. The process of angiogenesis takes place through the secretion of VEGF.[
Immunoexcitotoxicity, which is the interaction between inflammatory cytokines and glutamate receptors, is widely believed to have a significant impact on numerous neurological disorders and is currently being studied in relation to the growth and spread of tumors. Through its effects on metabolism, glutamate (a non-essential amino acid) plays a critical role in the progression of tumors. Activation of glutamate receptors can increase the effectiveness of effector T-cells or decrease cytokine production in immunosuppressive myeloid-derived suppressor cells, thereby enhancing antitumor immune responses. Inflammatory hematology ratios such as NLR, MLR, and LMR are believed to reflect the balance between pro-tumor inflammatory responses and antitumor immune responses. However, the specific relationship between immunoexcitotoxicity, glutamate, and the hematological parameters in the context of tumor growth and invasion needs further research for a more comprehensive understanding.[
Although preoperative hematological markers may help in grade prediction and prognosis in the clinical setting, the results of this meta-analysis have several limitations and require further analysis. First, studies that discuss the role of inflammatory markers in meningioma are still limited and only involve retrospective studies. Studies with other designs and involving more samples are needed to strengthen the result. Second, the samples used in each study mostly included low-grade meningiomas rather than high-grade meningiomas. Third, hematological marker values can be affected by the presence of infection, comorbid diseases, and medication use, which can affect the inflammatory parameter values in each study. Finally, the studies included in this meta-analysis have varying quality assessments, including studies with a high risk of bias. The results obtained in this meta-analysis suggest that more studies should be conducted to validate its conclusions. Furthermore, studies with more robust study designs and larger sample sizes, such as multicenter randomized controlled trials (RCTs), are needed.
CONCLUSION
Preoperative hematological inflammatory markers, particularly NLR, MLR, and LMR, can serve as potential predictors of meningioma grade. These findings provide valuable insights into the role of inflammatory markers as predictors of meningioma grade. These markers can be obtained easily and cost-effectively from routine preoperative laboratory tests. They can provide valuable information for clinicians in determining the prognosis and treatment plan for meningioma patients. However, further research and validation studies are needed to confirm these findings and establish the clinical utility of these inflammatory markers in predicting meningioma grade. Nonetheless, the meta-analysis provides valuable insights into the potential role of preoperative hematological inflammatory markers in meningioma management and prognosis.
Ethics approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ashwath KG, Aggarwal A, Praneeth K, Singla N, Gupta K. Neutrophil-to-lymphocyte ratio: Can it be used as an adjunct tool to predict histopathological grade of brain tumor?. J Neurosci Rural Pract. 2019. 10: 648-52
2. Blaylock RL. Immunoexcitatory mechanisms in glioma proliferation, invasion and occasional metastasis. Surg Neurol Int. 2013. 4: 15
3. Cho M, Joo JD, Kim IA, Han JH, Oh CW, Kim CY. The role of adjuvant treatment in patients with high-grade meningioma. 2017. 60: 527-33
4. Cohen JT, Miner TJ, Vezeridis MP. Is the neutrophil-tolymphocyte ratio a useful prognostic indicator in melanoma patients?. Melanoma Manag. 2020. 7: MMT47
5. de Oliveira Silva CB, Araújo B, Ongaratti BR, dos Santos TM, Rech CG, Coutinho LB. Preoperative hematological inflammatory markers associated with grade and survival in Meningiomas. Surg Exp Pathol. 2022. 5: 5
6. Ding Y, Qiu L, Xu Q, Song L, Yang S, Yang T. Relationships between tumor microenvironment and clinicopathological parameters in meningioma. Int J Clin Exp Pathol. 2014. 7: 6973-9
7. El-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol. 1987. 139: 2406-13
8. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006. 313: 1960-4
9. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br J Cancer. 2011. 105: 93-103
10. Gregory AD, Houghton AM. Tumor-associated neutrophils: New targets for cancer therapy. Cancer Res. 2011. 71: 2411-6
11. Greten FR, Grivennikov SI. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity. 2019. 51: 27-41
12. Guidry BS, Chotai S, Tang AR, Le CH, Grisham CJ, McDermott JR. Association between preoperative hematologic markers and aggressive behavior in meningiomas. Clin Neurol Neurosurg. 2023. 226: 107629
13. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011. 144: 646-74
14. Huntoon K, Toland AM, Dahiya S. Meningioma: A review of clinicopathological and molecular aspects. Front Oncol. 2020. 10: 579599
15. Iwase R, Shiba H, Haruki K, Fujiwara Y, Furukawa K, Futagawa Y. Post-operative lymphocyte count may predict the outcome of radical resection for gallbladder carcinoma. Anticancer Res. 2013. 33: 3439-44
16. Jarmuzek P, Kozlowska K, Defort P, Kot M, Zembron-Lacny A. Prognostic values of systemic inflammatory immunological markers in glioblastoma: A systematic review and meta-analysis. Cancers (Basel). 2023. 15: 3339
17. Kalamarides M, Peyre M, editors. An overview of meningiomas. Meningiomas: Comprehensive strategies for management. Germany: Springer Nature; 2020. p. 3-10
18. Kayhan A, Korkmaz TS, Baran O, Kemerdere R, Yeni SN, Tanriverdi T. Preoperative systemic inflammatory markers in different brain pathologies: An analysis of 140 patients. Turk Neurosurg. 2019. 29: 799-803
19. Kemerdere R, Akgun MY, Toklu S, Alizada O, Tanriverdi T. Preoperative systemic inflammatory markers in low-and high-grade gliomas: A retrospective analysis of 171 patients. Heliyon. 2019. 5: e01681
20. Khanzadeh S, Azarhomayoun A, Rahmati R, Meidani FZ, Baughn C, Clark A. Neutrophil-to-lymphocyte ratio as an effective biomarker for meningioma: A systematic review and meta-analysis. Explor Res Hypothesis Med. 2023. 00: 1-10
21. Khayat Kashani HR, Azhari S, Nayebaghayee H, Salimi S, Mohammadi HR. Prediction value of preoperative findings on meningioma grading using artificial neural network. Clin Neurol Neurosurg. 2020. 196: 105947
22. Lei YY, Li YT, Hu QL, Wang J, Sui AX. Prognostic impact of neutrophil-to-lymphocyte ratio in gliomas: A systematic review and meta-analysis. World J Surg Oncol. 2019. 17: 152
23. Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Int J Cancer. 2014. 134: 2403-13
24. Liang RF, Li M, Li JH, Zuo MR, Yang Y, Liu YH. The significance of preoperative hematological inflammatory markers in patients with meningiomas. Clin Neurol Neurosurg. 2019. 182: 1-4
25. Lin M, Hu T, Yan L, Xiao D, Zhao H, Yan P. Can systemic inflammatory markers be used to predict the pathological grade of meningioma before surgery?. World Neurosurg. 2019. 127: e677-84
26. Lin Y, Dai P, Lin Q, Chen J. Predictive nomogram for atypical meningioma based on preoperative magnetic resonance imaging and routine blood tests. World Neurosurg. 2022. 163: e610-6
27. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
28. Luo H, He L, Zhang G, Yu J, Chen Y, Yin H. Normal reference intervals of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and systemic immune inflammation index in healthy adults: A large multi-center study from Western China. Clin Lab. 2019. 65: 255-65
29. Maggio I, Franceschi E, Tosoni A, Di Nunno V, Gatto L. Meningioma: Not always a benign tumor. A review of advances in the treatment of meningiomas. CNS Oncol. 2021. 10: CNS72
30. Manjunath N, Mishra S, Garg K, Suri V, Sharma MC, Tandon V. Is there any relationship between systemic inflammatory markers and meningioma grade?. Neurol India. 2022. 70: 223-30
31. Massara M, Persico P, Bonavita O, Poeta VM, Locati M, Simonelli M. Neutrophils in gliomas. Front Immunol. 2017. 8: 1349
32. Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol. 2019. 9: 1146
33. Misiewicz A, Dymicka-Piekarska V. Fashionable, but what is their real clinical usefulness? NLR, LMR, and PLR as a promising indicator in colorectal cancer prognosis: A systematic review. J Inflamm Res. 2023. 16: 69-81
34. Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neurooncology. 2022. 24: 1-95
35. Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol. 1985. 134: 230-4
36. Pirozzolo G, Gisbertz SS, Castoro C, van Berge Henegouwen MI, Scarpa M. Neutrophil-to-lymphocyte ratio as prognostic marker in esophageal cancer: A systematic review and meta-analysis. J Thorac Dis. 2019. 11: 3136-45
37. Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015. 122: 4-23
38. Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J. Tumor-associated neutrophils as a new prognostic factor in cancer: A systematic review and meta-analysis. PLoS One. 2014. 9: e98259
39. Song Q, Wu JZ, Wang S. Low preoperative lymphocyte to monocyte ratio serves as a worse prognostic marker in patients with esophageal squamous cell carcinoma undergoing curative tumor resection. J Cancer. 2019. 10: 2057-62
40. Song X, Zhang H, Yin F, Guo P, Yang X, Liu J. Systemic inflammatory markers for predicting overall survival in patients with osteosarcoma : A systematic review and meta-analysis. Mediators Inflamm. 2021. 2021: 3456629
41. Szor DJ, Dias AR, Pereira MA, Ramos MF, Zilberstein B, Cecconello I. Prognostic role of neutrophil/lymphocyte ratio in resected gastric cancer: A systematic review and meta-analysis. Clinics (Sao Paulo). 2018. 73: e360
42. Uribe-Querol E, Rosales C. Neutrophils in cancer: Two sides of the same coin. J Immunol Res. 2015. 2015: 983698
43. Wan L, Wu C, Luo S, Xie X. Prognostic value of lymphocyte-tomonocyte ratio (LMR) in cancer patients undergoing immune checkpoint inhibitors. Dis Markers. 2022. 2022: 3610038
44. Wang PF, Meng Z, Song HW, Yao K, Duan ZJ, Yu CJ. Preoperative changes in hematological markers and predictors of glioma grade and survival. Front Pharmacol. 2018. 9: 886
45. Wei B, Yao M, Xing C, Wang W, Yao J, Hong Y. The neutrophil lymphocyte ratio is associated with breast cancer prognosis: An updated systematic review and meta-analysis. Onco Targets Ther. 2016. 9: 5567-75
46. Weng W, Chen X, Gong S, Guo L, Zhang X. Preoperative neutrophil-lymphocyte ratio correlated with glioma grading and glioblastoma survival. Neurol Res. 2018. 40: 917-22
47. Xiang J, Zhou L, Li X, Bao W, Chen T, Xi X. Preoperative monocyte-to-lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancer. Transl Oncol. 2017. 10: 33-9
48. Xu B, Chen Z, Zhang J, Chang J, Zhao W, Dong Z. Prognostic value of peripheral whole blood cell counts derived indexes in gallbladder carcinoma: A systematic review and meta-analysis. Front Oncol. 2021. 11: 707742
49. Zhang Z, Wang S, Ren F, Yang L, Xie H, Pan L. Inflammatory factors and risk of meningiomas: A bidirectional mendelian-randomization study. Front Neurosci. 2023. 17: 1186312
50. Zhu Y, Zhou S, Liu Y, Zhai L, Sun X. Prognostic value of systemic inflammatory markers in ovarian Cancer: A PRISMA-compliant meta-analysis and systematic review. BMC Cancer. 2018. 18: 443
51. Zuo MR, Liang RF, Li M, Xiang YF, Zhang SX, Yang Y. A comprehensive study of risk factors for post-operative pneumonia following resection of meningioma. BMC Cancer. 2019. 19: 100