- Department of Neurosurgery, Fundacion Santa Fe de Bogota, Bogota, Colombia
- Department of Neurosurgery, High Complexity Clinic of the Caribbean, Valledupar, Colombia
- Faculty of Medicine, Universidad El Bosque, Bogota, Colombia.
Correspondence Address:
Juan Armando Mejia, Department of Neurosurgery, Fundacion Santa Fe de Bogota, Bogota, Colombia.
DOI:10.25259/SNI_545_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Juan Armando Mejia1, Maximiliano Paez Nova2, Luis Garcia Rairan3. The rule of five: A novel anatomical landmark for approaching cavernous sinus content. 28-Jul-2023;14:269
How to cite this URL: Juan Armando Mejia1, Maximiliano Paez Nova2, Luis Garcia Rairan3. The rule of five: A novel anatomical landmark for approaching cavernous sinus content. 28-Jul-2023;14:269. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12461
Abstract
Background: The main objective of this study is to enhance neurosurgeons’ anatomical knowledge by providing specific anatomical references of the cavernous sinus (CS). However, it is essential to clarify that our study does not seek to establish an absolute intraoperative rule due to the inherent anatomical variability that must be considered.
Methods: Fifty-three cadaveric specimens were procured from the Forensic Institute (Bogotá) and subjected to dissection through an extradural approach. The measurements were taken in two distinct phases. The first phase involved the measurement of various anatomical structures in 25 specimens with respect to the anterior and posterior clinoids. The second phase, which was conducted 5 years later, involved the measurement of the distance between the foramen rotundum and the foramen ovale in 28 specimens using the L&W tools microcaliper.
Results: In 25 specimens, a perpendicular imaginary line was drawn from the lateral tip of the anterior clinoid to the floor of the medial fossa. This facilitated access to the Parkinson’s triangle, which is located between the IV cranial nerve and the ophthalmic V1 nerve, revealing a constant distance of 5 mm between the lateral tip of the anterior clinoid and the IV cranial nerve. Furthermore, in 28 specimens, the mean distance from the foramen rotundum to the foramen ovale was found to be 1.3 cm bilaterally.
Conclusion: The rule of five is a valuable tool for comprehensively understanding the anatomy of the CS, providing a reference point for the different normal anatomical structures within the CS.
Keywords: Anatomy, Cavernous sinus, Cranial nerves, Microsurgical anatomy, Triangles
INTRODUCTION
The cavernous sinus (CS) is a complex and critical structure located at the base of the skull that houses several cranial nerves and blood vessels. Due to its central location and proximity to vital structures such as the internal carotid artery, the pituitary gland, and the optic chiasm, accessing the CS can be challenging and risky for neurosurgeons.[
Tumors and other pathologies affecting the CS can cause a variety of symptoms, including visual disturbances, diplopia, and cranial nerve palsies.[
Several anatomical landmarks can guide neurosurgeons in approaching the CS safely, such as the anterior and posterior clinoid processes and the foramen rotundum and ovale.[
Technological advancements in high-resolution imaging and endoscopic techniques have improved the visualization of the CS’s microanatomy, making surgical interventions more accurate and successful.[
The primary objective of this study is to provide valuable insights into safe and reliable anatomical references, with the intention of strengthening the anatomical knowledge of neurosurgeons. However, it is important to clarify that our study does not claim to establish an intraoperative rule due to the anatomical variability that may exist.
MATERIALS AND METHODS
The study was approved by the Ethics Committee of the Forensic Institute of Bogotá following the principles of the Declaration of Helsinki. Dissections were performed on cadavers from the Institute of Forensic Medicine and Forensic Sciences, and specimens were selected based on specific criteria. Cadavers were chosen that had died within <4 h, had not died due to gunshot wounds to the head or blunt force trauma cranioencephalic, had not died due to hanging or strangulation, and had not died due to neurological causes or skull base fractures. In addition, cadavers with facial fractures were not included in the study.
The study was conducted in two phases, in the first phase, which was conducted earlier, measurements were taken on 25 cadaver specimens. Later, in the second phase, measurements were taken on 28 specimens. The reason for conducting the study in two phases was that during the first phase, there was an interest in measuring specific structures, and subsequently, the interest emerged in measuring the distance between the foramen rotundum and foramen ovale. However, due to the unavailability of cadavers, the study was delayed significantly.
The dissections were performed through a bicoronal incision, followed by craniotomy of the calvaria, including the pterional and temporal regions, and the brains were removed with a section of cranial nerves attached for full viewing.
To ensure accurate and precise measurements, all measurements were collected using the L&W tools microcaliper, a highly precise instrument capable of measuring with an accuracy of up to 0.01 mm.
In the first phase, the following measurements were taken on 25 cadaver specimens:
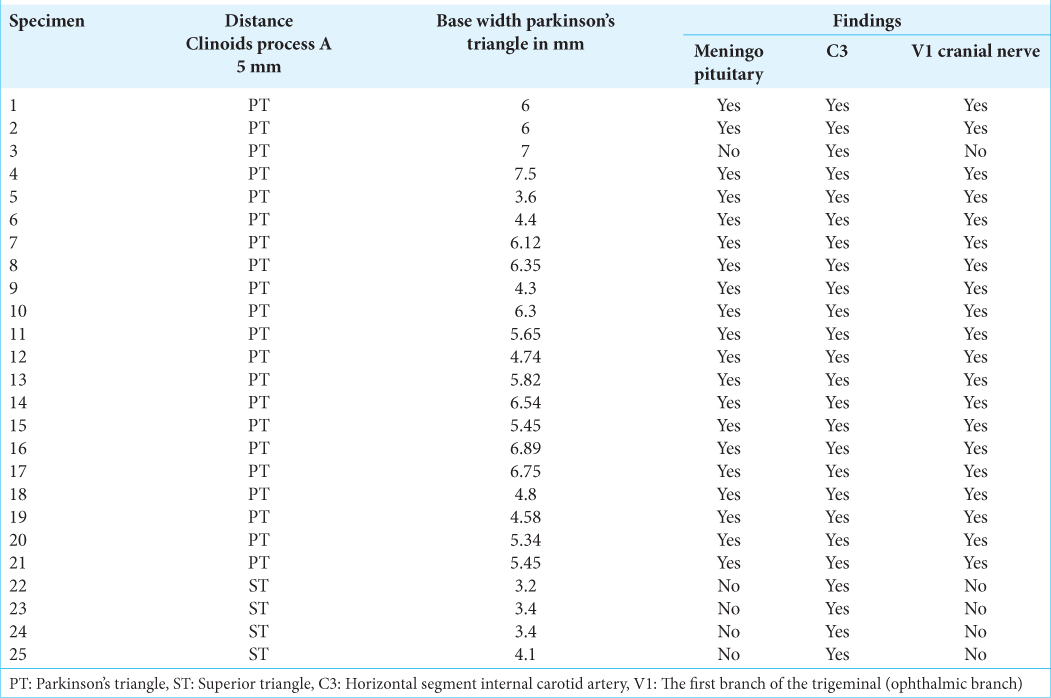
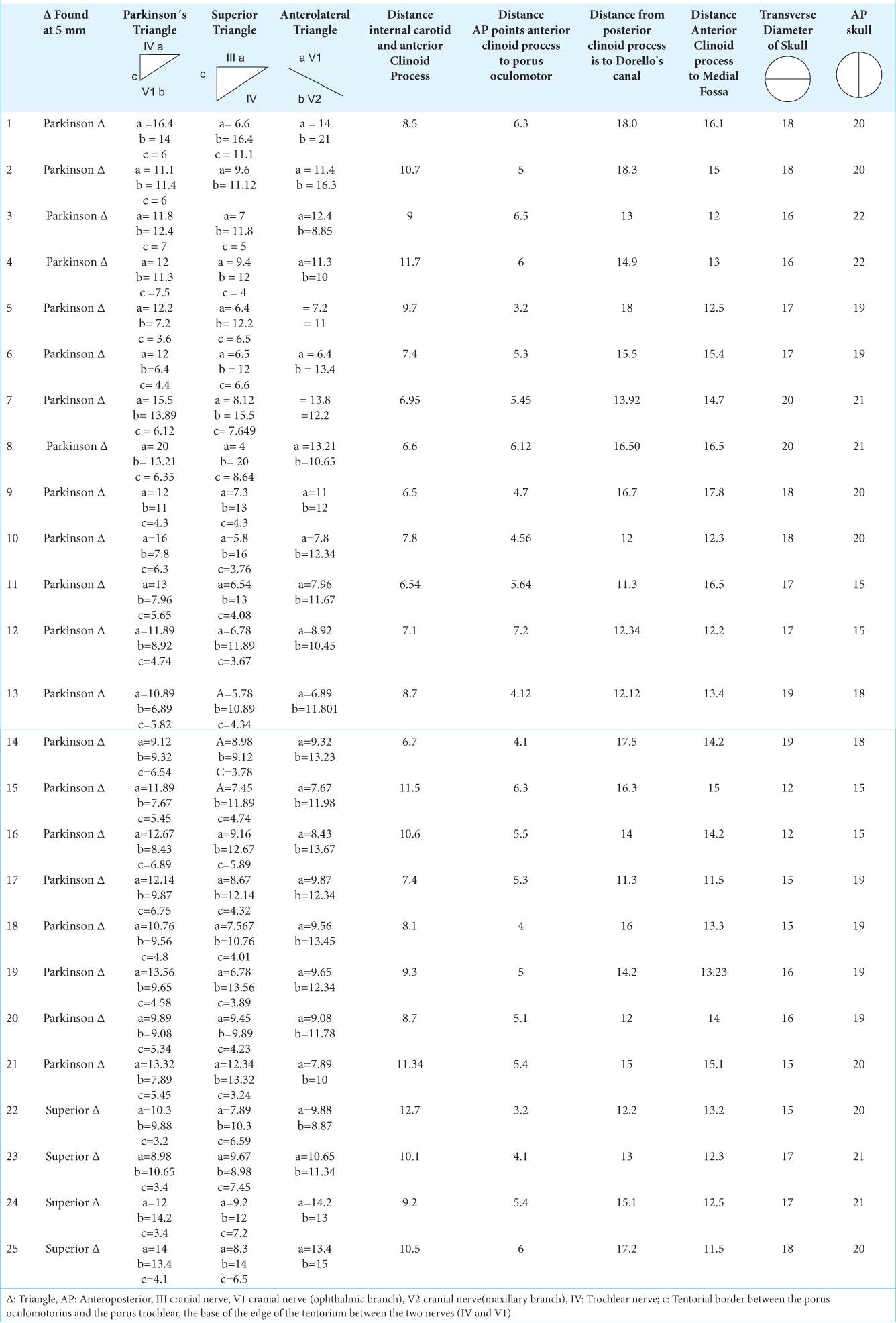
Line perpendicularly joining the lateral tip of the anterior clinoid process with the floor of the middle fossa Triangle found 5 mm from the line mentioned above Length of the edges of the superior triangle, Parkinson’s triangle, and anterolateral triangle Distance from the lateral tip of the anterior clinoid process to the medial aspect of the internal carotid artery as it passes from the region subclinoid to supraclinoid Anteroposterior distance from the lateral tip of the clinoid process anterior to oculomotor porus For the distance from the posterior clinoid process to the origin of the Dorello’s canal, five specimens were used out of the 25 specimens chosen Transverse and anteroposterior diameters of the skulls studied (measured between the intracranial face on one side and the intracranial face on the other side) In five specimens, measurements were made between the posterior clinoid process and Dorello’s canal.
In the second phase, the following measurements were taken on 28 specimens:
Distance between the foramen rotundum to the foramen ovale.
The measurements were recorded on three separate occasions, and the mean value of each measurement was taken as the true value. In addition, all measurements collected in both phases were recorded in an Excel program, which facilitated the calculation of means and allowed for the detection of any differences among the measurements of each structure. This method provided a convenient and efficient way to visualize the data and generate graphs and charts, enabling a more comprehensive presentation of the results.
RESULTS
The dissection of 25 specimens [
The measurements of Parkinson’s triangle were as follows: the fourth cranial nerve (side a) measured 12.53 mm, V-1 cranial nerve (side b) measured 10.07 mm, and the width (side c) measured 5.3 mm. The cranial nerves were measured using a perpendicular line to the floor of the middle fossa that falls from the lateral tip of the anterior clinoid process as a reference, and their length was measured until they intersected with other cranial nerves to form the triangles.
Moreover, the average distance from the medial aspect of the anterior clinoid process to the medial aspect of the emergence of the internal carotid artery from subclinoid to supraclinoidea was 8.93 mm [
Furthermore, the measurements of the anteroposterior and transversus axis of the skull were taken without the thickness of the diploe, providing a comparison parameter [
CS triangles
The photographs of the anatomical dissections provide a comprehensive view of the microsurgical anatomy of the CS.
Figure 1:
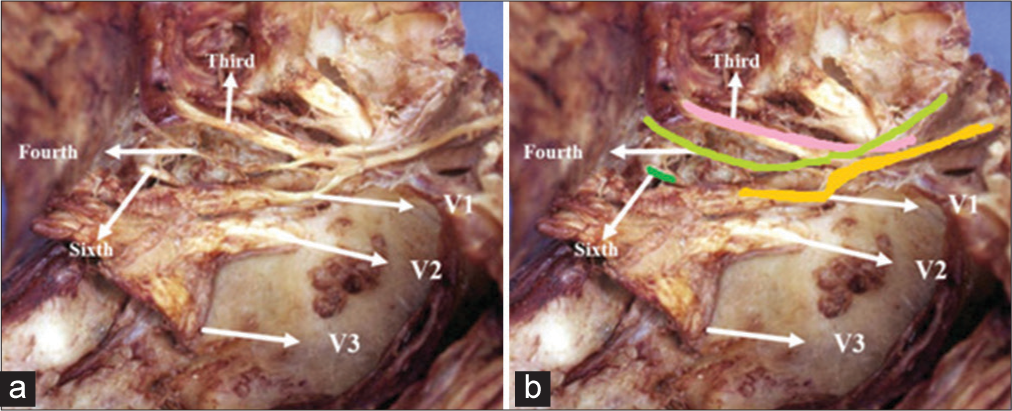
(a and b) Main anatomical repairs present in the cavernous sinus. The image shows the relationship of the differente cranial nerves with each other a) Sixth cranial nerve, fourth cranial nerve, third cranial nerve, V1 cranial nerve, V2 cranial nerve, V3 cranial nerve. b) sixth cranial nerve, fourth cranial nerve, third cranial nerve, V1 cranial nerve, V2 cranial nerve, V3 cranial nerve.
Figure 3:
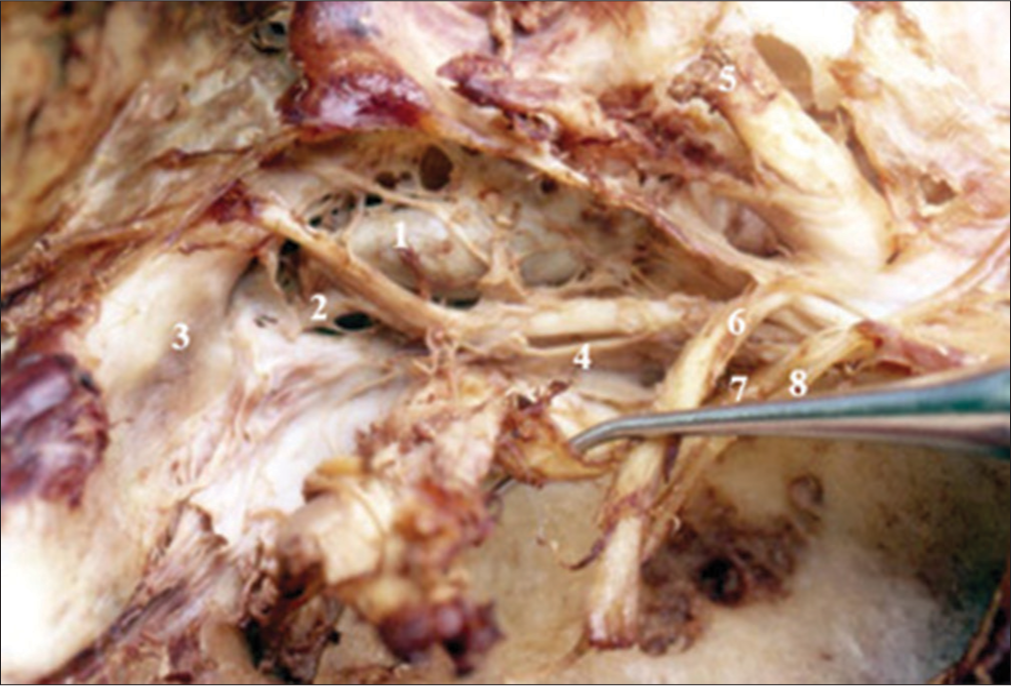
Superior posterior and medial compartments of the right cavernous sinus. 1-Medial compartment (Cavernous internal carotid artery), 2-Posterior compartment, 3-Membrane of anterior foramen lacerate, 4-VI cranial nerve, 5-Optic nerve, 6-III cranial nerve, 7-IV cranial nerve, and 8-V-1 cranial nerve retracted with the dissector forward.
Figure 4:
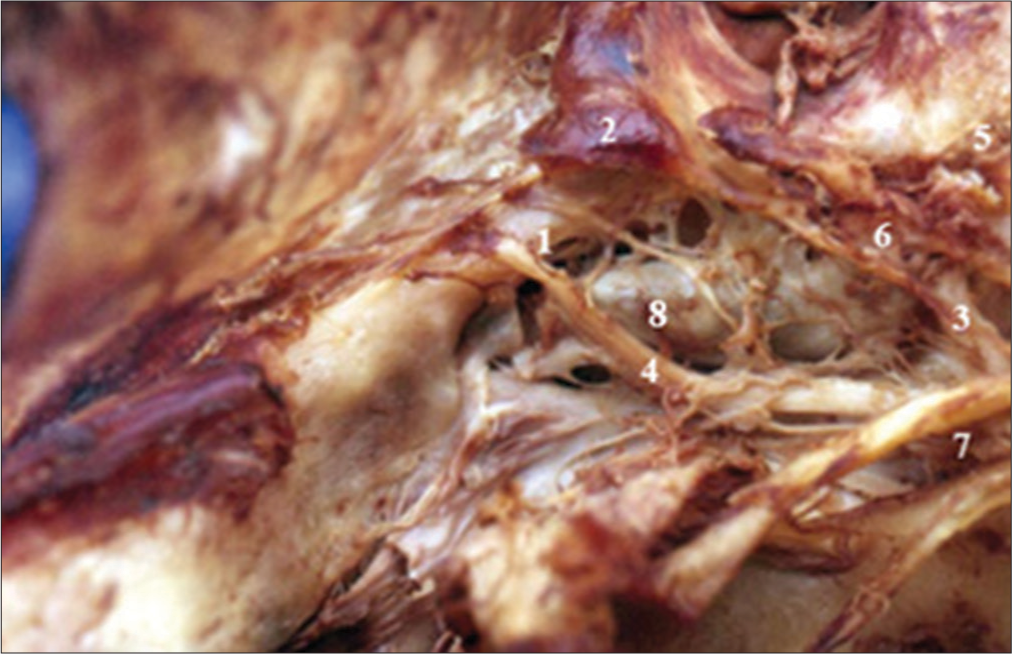
Vascular trunks dependent on the intracavernous A. Right internal carotid intracavernous. 1-Meningo-hypophyseal trunk area, 2-Area of the Meningeal branch Dorsal, 3-Area of the inferolateral trunk, 4-VI cranial nerve, 5-Supraclinoid segment, 6-Subclinoid segment, 7-III, IV and V-1 retracted cranial nerve, and 8-Intracavernous internal carotid artery.
Figure 5:
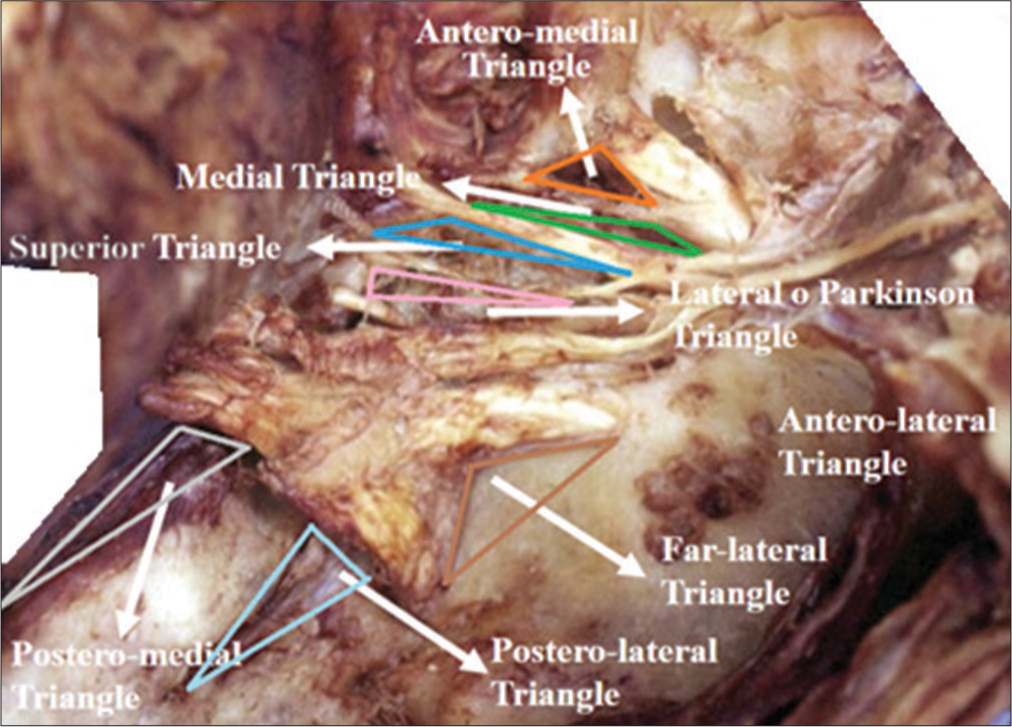
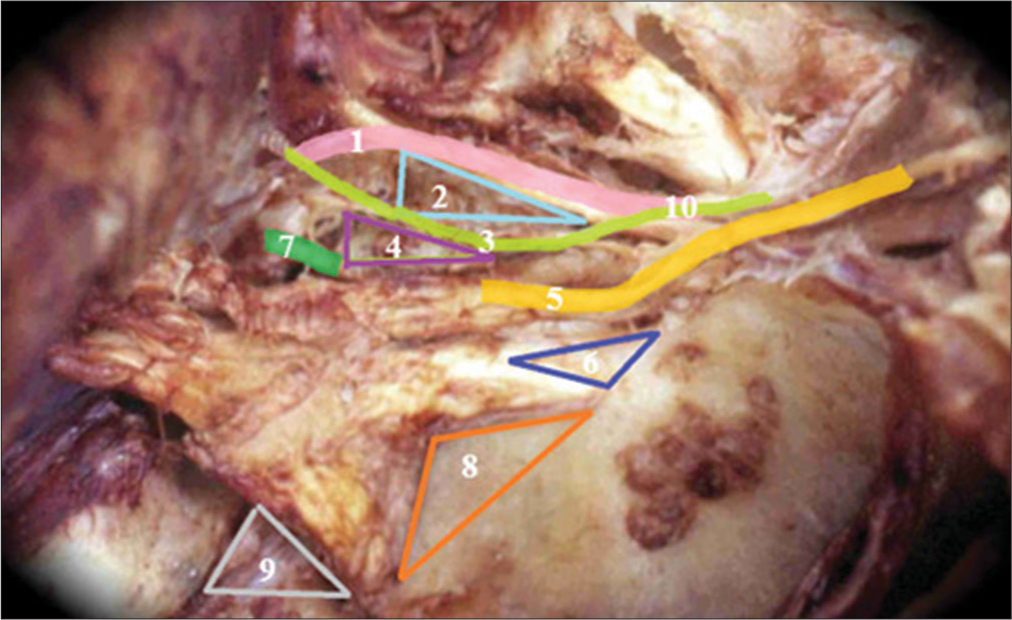
Triangles of the lateral wall of the cavernous sinus right. 1-III cranial nerve, 2-Superior triangle, 3-IV cranial nerve, 4-Parkinson triangle, 5-V-1, 6-Anterolateral triangle, 7-Sixth cranial nerve, 8-Extreme lateral Triangle, 9-Glassock’s triangle, and 10-Crossing of the IV cranial nerve over the III cranial nerve.
The fourth cranial nerve and Parkinson’s triangle are vertically positioned approximately 5 mm from the lateral tip of the anterior clinoid process, while the foramen rotundum is located approximately 5 mm away from the fourth cranial nerve. In addition, there is a consistent distance of approximately 5 mm from the foramen rotundum to the ovale. Moreover, Dorello’s canal, through which the sixth cranial nerve passes, is situated 15 mm posteriorly from the posterior clinoid process, as outlined in
DISCUSSION
Our study highlights the significance of acquiring a comprehensive understanding of the topographic anatomy of the CS to enhance the anatomical knowledge of neurosurgeons. However, it is important to note that our findings do not serve as an intraoperative anatomical rule due to the potential deformations of the CS caused by various pathologies.[
This study introduced the rule of five, which emphasizes the precise localization of various anatomical structures within the CS. These structures include the fourth cranial nerve (upper limit of Parkinson’s triangle), Parkinson’s triangle, the foramen rotundum, the foramen ovale, and Dorello’s canal through which the sixth nerve passes. The location of these structures is determined with reference to specific bony landmarks.
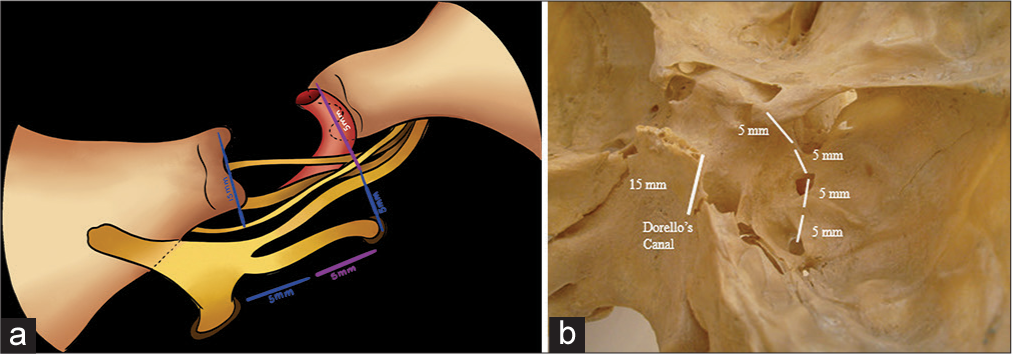
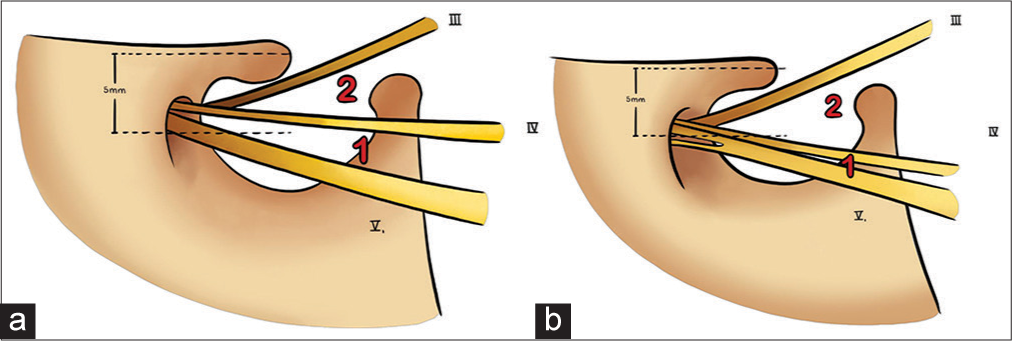
The “Rule of five” [
Figure 6:
The rule of five stablishes that 5 mm from the clinoid process in most cases is the Parkinson’s triangle, 5 mm from this triangle is the rotundum foramen, and 5 mm from this last is the foramen ovale. (a) It shows the nerve relationships of the foramen rotundum and foramen ovale. V2 exits through the foramen rotundum and V3 exits through the foramen ovale, and the VI cranial nerve passes through Dorello’s canal, (b) represents the rule of five and its bone relationship.
Furthermore, it has been found that the III cranial nerve is situated approximately 10 mm from the IV cranial nerve. However, some studies, including the one conducted by Watanabe et al., have found the distance to be around 9.6 ± 3.6 mm.[
Similarly, this study has determined that Dorello’s canal, through which the sixth cranial nerve passes, is positioned 15 mm posteriorly from the posterior clinoid process.
The location of Parkinson’s triangle can be determined by utilizing the anterior clinoid process as a reference point. By drawing an imaginary line perpendicular to the foramen rotundum from the lateral tip of the anterior clinoid process, the triangle can be assessed. Research findings indicate that in 86% of cases, Parkinson’s triangle is situated 5 mm away from the lateral tip of the anterior clinoid process, while only 14% exhibit the superior triangle [
Figure 7:
Relation between Parkinson’s triangle area (1) versus superior triangle (2) to 5 vertical mm from the lateral tip of the clinoid process. (a) In 86% of the cases, the Parkinson’s triangle is located 5 mm from the anterior clinoid process, (b) The remaining 14% is the superior triangle. (a and b) III cranial nerve, IV cranial nerve and V1 cranial nerve.
Parkinson’s triangle plays a crucial role in neurosurgery, as it serves as an important landmark for accessing CS. The upper boundary of Parkinson’s triangle is defined by the fourth cranial nerve, and its identification is imperative to avoid damage during surgical procedures, which can result in impaired eye movement. Furthermore, this triangle is also relevant to other structures such as the oculomotor nerve and the posterior cerebral artery, making it a valuable reference point for various neurosurgical interventions.[
Strengths
This research presents significant contributions to our understanding of the anatomical landmarks within the CS through the utilization of a simple and practical “rule of five.” The identification of Parkinson’s triangle, which facilitates the localization of the thinner cranial nerve (fourth cranial nerve), along with the foramen rotundum, foramen ovale, and Dorello’s canal, serves as a valuable reference for obtaining detailed knowledge of the CS.
Furthermore, the research emphasizes the importance of recognizing normal anatomical structures, despite the fact that various tumors and abnormalities can distort the CS’s normal structure. This approach provides a point of reference during surgical interventions, which can help minimize complications and reduce patient morbidity.
Limitations and future research
Regarding the limitations of this study, it is paramount to acknowledge that pathologies causing significant deformations of the CS were not considered. Therefore, the parameters established in this research solely serve as an anatomical reference, and their surgical applicability is limited due to the inherent variability of the CS, the potential deformations caused by pathologies, and the necessity of clinoidectomy in surgical approaches.
Further investigations may also consider variations in the transverse and anteroposterior diameters of the skull, as these factors can impact the heights and distances of specific portions within the cranial vault. Such studies could enhance the current research by accounting for deformities in the normal anatomy of the CS, thus providing surgeons with a more comprehensive understanding of the challenges posed by various pathologies. These considerations may facilitate the development of more refined and effective surgical interventions in the future.
CONCLUSION
This study anatomically dissects the CS to enhance the knowledge of neurosurgeons and facilitate the acquisition of CS-specific expertise among trainee neurosurgeons. It is important to note that the primary objective of this research is not to offer surgical guidance, but rather to establish a comprehensive reference for comprehending the intricate normal anatomy of the CS.
Declaration of patient consent
The Institutional review board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ajisebutu A, Del Bigio MR, Kazina CJ, West M, Serletis D. Dr. Dwight Parkinson: A Canadian neurosurgical pioneer. J Neurosurg. 2019. 133: 1092-9
2. Altay T, Patel BC, Couldwell WT. Lateral orbital wall approach to the cavernous sinus: Laboratory investigation. J Neurosurg. 2012. 116: 755-63
3. Alves RV, Sousa LC, Rodrigues JP, Laube KA. Revisiting Parkinson: After six decades, his triangle remains useful. Surg Neurol Int. 2022. 13: 483
4. Balcerzak A, Tubbs RS, Zielinska N, Olewnik Ł. Clinical analysis of cavernous sinus anatomy, pathologies, diagnostics, surgical management and complications-Comprehensive review. Ann Anat. 2023. 245: 152004
5. Chotai S, Liu Y, Qi S. Review of surgical anatomy of the tumors involving cavernous sinus. Asian J Neurosurg. 2018. 13: 1-8
6. DeMonte F, Diaz E, Callender D, Suk I. Transmandibular, circumglossal, retropharyngeal approach for chordomas of the clivus and upper cervical spine. Technical note. Neurosurg Focus. 2001. 10: E10
7. Diaz FG, Ohaegbulam S, Dujovny M, Ausman JI. Surgical alternatives in the treatment of cavernous sinus aneurysms. J Neurosurg. 1989. 71: 846-53
8. Dolci RL, Upadhyay S, Filho LF, Fiore ME, Buohliqah L, Lazarini PR. Endoscopic endonasal study of the cavernous sinus and quadrangular space: Anatomic relationships. Head Neck. 2016. 38: E1680-7
9. González-Darder JM, Capilla-Guasch P. Microsurgical technique for en bloc anatomical exenteration of cavernous sinus compartment to treat invasive meningioma. Interdiscipl Neurosurg. 2022. 30: 101646
10. Isolan GR, Braga FL, Campero A, Landeiro JA, de Araújo RM, Adjer P. Microsurgical and endoscopic anatomy of the cavernous sinus. Arq Brasil Neurocir. 2020. 39: 83-94
11. Kına H, Ayran A, Demirtaş İ, editors. Microsurgical anatomy of the cavernous sinus and limitations of surgical approaches: A cadaveric study. Folia Morphol (Warsz). 2022. p.
12. Parkinson D. A surgical approach to the cavernous portion of the carotid artery: Anatomical studies and case report. J Neurosurg. 1965. 23: 474-83
13. Ulutas M, Boyacı S, Akakın A, Kılıç T, Aksoy K. Surgical anatomy of the cavernous sinus, superior orbital fissure, and orbital apex via a lateral orbitotomy approach: A cadaveric anatomical study. Acta Neurochir (Wien). 2016. 158: 2135-48
14. Volovici V, Dammers R. How I do it: Proximal control in Parkinson’s triangle for a very large paraclinoid aneurysm. Acta Neurochir (Wien). 2021. 163: 2967-71
15. Watanabe A, Nagaseki Y, Ohkubo S, Ohhashi Y, Horikoshi T, Nishigaya K. Anatomical variations of the ten triangles around the cavernous sinus. Clin Anat. 2003. 16: 9-14
16. Yang Y, Zhan G, Liao J, Dang R, Wang H, Li Y. Morphological characteristics of the sphenoid sinus and endoscopic localization of the cavernous sinus. J Craniofac Surg. 2015. 26: 1983-7
17. Zhang P, Xi H, Li W. The clinical anatomy of the cavernous sinus. Forensic Med Anat Res. 2015. 3: 66-75